当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Oxygen Exchange in Mixed Ionic-Electronic Conductors: A Current–Voltage Study
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.chemmater.4c02732 Di Chen, Zixuan Guan, Hongyang Su, Wenle Yan, Chang Liu, Hanshi Li, Dawei Zhang, William C. Chueh
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.chemmater.4c02732 Di Chen, Zixuan Guan, Hongyang Su, Wenle Yan, Chang Liu, Hanshi Li, Dawei Zhang, William C. Chueh
|
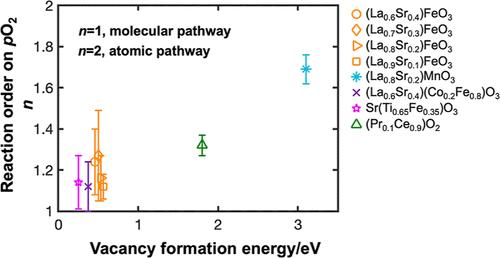
The oxygen incorporation reaction at solid/gas interfaces is central to many critical processes in solid-state electrochemistry, including those in electrolytic and thermochemical water splitting, fuel cells, oxygen storage for emissions control, and permeation membranes. To understand the reaction pathway and identify the rate-determining step, near-equilibrium measurements have been employed to quantify the exchange coefficients as a function of oxygen partial pressure and temperature. However, because the exchange coefficient contains contributions from both forward and reverse reaction rate constants and depends on the oxygen gas partial pressure (pO2) and oxygen fugacity (pO2,eff) in the solid, unique and definitive mechanistic assessment has been challenging. In this study, we addressed this challenge by measuring the current–voltage curve at far-from-equilibrium conditions for several mixed ionic–electronic conductors, including the (La,Sr)FeO3−δ family, (La,Sr)(Fe,Co)O3−δ, Sr(Ti,Fe)O3−δ, (La,Sr)MnO3−δ, and (Pr,Ce)O2−δ, which are commonly used as cathode materials for solid-oxide fuel cells. We isolated the driving forces acting in the gaseous and solid phases by independently controlling pO2 and pO2,eff, respectively, and a kinetics model was successfully applied. Key parameters, including the reaction orders with respect to pO2 and pO2,eff, were extracted and correlated to the reaction pathway. Further analysis showed that the vacancy formation energy is a possible descriptor for the reaction pathways.
中文翻译:

混合离子-电子导体中氧交换的机理见解:电流-电压研究
固/气界面的氧掺入反应是固态电化学中许多关键过程的核心,包括电解和热化学水分解、燃料电池、用于排放控制的储氧和渗透膜中的过程。为了了解反应途径并确定速率确定步骤,采用了近平衡测量来量化交换系数作为氧分压和温度的函数。然而,由于交换系数包含来自正向和反向反应速率常数的贡献,并且取决于固体中的氧气分压 (pO2) 和氧逸度 (pO2,eff),因此独特和确定的机理评估一直具有挑战性。在这项研究中,我们通过测量几种混合离子-电子导体在远非平衡条件下的电流-电压曲线来应对这一挑战,包括 (La,Sr)FeO3-δ 族、(La,Sr)(Fe,Co)O3-δ、Sr(Ti,Fe)O3-δ、(La,Sr)MnO3-δ 和 (Pr,Ce)O2-δ,它们通常用作固体氧化物燃料电池的正极材料。我们通过分别独立控制 pO2 和 pO2,eff 来分离作用在气相和固相中的驱动力,并成功应用了动力学模型。提取关键参数,包括关于 pO2 和 pO2,eff 的反应顺序,并与反应途径相关联。进一步分析表明,空位形成能是反应途径的可能描述符。
更新日期:2024-11-20
中文翻译:

混合离子-电子导体中氧交换的机理见解:电流-电压研究
固/气界面的氧掺入反应是固态电化学中许多关键过程的核心,包括电解和热化学水分解、燃料电池、用于排放控制的储氧和渗透膜中的过程。为了了解反应途径并确定速率确定步骤,采用了近平衡测量来量化交换系数作为氧分压和温度的函数。然而,由于交换系数包含来自正向和反向反应速率常数的贡献,并且取决于固体中的氧气分压 (pO2) 和氧逸度 (pO2,eff),因此独特和确定的机理评估一直具有挑战性。在这项研究中,我们通过测量几种混合离子-电子导体在远非平衡条件下的电流-电压曲线来应对这一挑战,包括 (La,Sr)FeO3-δ 族、(La,Sr)(Fe,Co)O3-δ、Sr(Ti,Fe)O3-δ、(La,Sr)MnO3-δ 和 (Pr,Ce)O2-δ,它们通常用作固体氧化物燃料电池的正极材料。我们通过分别独立控制 pO2 和 pO2,eff 来分离作用在气相和固相中的驱动力,并成功应用了动力学模型。提取关键参数,包括关于 pO2 和 pO2,eff 的反应顺序,并与反应途径相关联。进一步分析表明,空位形成能是反应途径的可能描述符。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号