当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
La0.4Sr0.6CoO3-Catalyzed Selective Oxidation of Ethylbenzene to Acetophenone without Solvent: A New Reactive Oxygen Species Transformation Mechanism Mediated by •O2– Derived from 1O2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-20 , DOI: 10.1021/acssuschemeng.4c06775 Weiwen Mao, Jiaheng Qin, Miao Li, Ming Chen, Wangyu Fu, Zongyan Ma, Jing Chen, Tongtong Fan, Yu Long, Jiantai Ma
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-20 , DOI: 10.1021/acssuschemeng.4c06775 Weiwen Mao, Jiaheng Qin, Miao Li, Ming Chen, Wangyu Fu, Zongyan Ma, Jing Chen, Tongtong Fan, Yu Long, Jiantai Ma
|
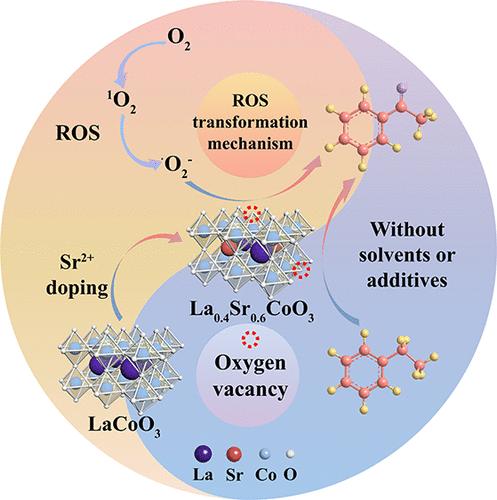
Developing a green, stable, and cost-effective heterogeneous catalyst and clarifying its catalytic mechanism for the selective oxidation of C–H bonds without solvent to carbonyl compounds hold a significant theoretical and practical value. Herein, we synthesize perovskite catalysts using the sol–gel method to catalyze the selective oxidation of ethylbenzene. Notably, La0.4Sr0.6CoO3-800 (800 °C is the calcination temperature of the catalyst) demonstrates remarkable efficacy, converting 73% of ethylbenzene into acetophenone with a selectivity of 93%. Characterization analyses reveal that the incorporation of strontium moderately disrupts the internal balance of the perovskite structure, leading to increased oxygen vacancies and enhanced oxygen adsorption capacity. Moreover, electron paramagnetic resonance and mechanistic studies prove that molecular oxygen on the catalyst surface is converted to singlet oxygen (1O2) and superoxide radical anions (•O2–). The presence of 1O2 significantly aids in the production of •O2–, thereby effectively promoting the oxidation of ethylbenzene. This research introduces a new reactive oxygen species (ROS) transformation mechanism and provides valuable insights into the selective oxidation of hydrocarbons.
中文翻译:

La0.4Sr0.6CoO3催化的乙苯无溶剂选择性氧化制苯乙酮:一种由•O2–衍生的活性氧转化机制
开发一种绿色、稳定且具有成本效益的非均相催化剂,并阐明其在没有溶剂的情况下将 C-H 键选择性氧化成羰基化合物的催化机制具有重要的理论和实践价值。在此,我们使用溶胶-凝胶法合成钙钛矿催化剂,以催化乙苯的选择性氧化。值得注意的是,La0.4Sr0.6CoO 3-800(800 °C 是催化剂的煅烧温度)表现出显着的功效,将 73% 的乙苯转化为苯乙酮,选择性为 93%。表征分析表明,锶的掺入适度破坏了钙钛矿结构的内部平衡,导致氧空位增加和氧吸附能力增强。此外,电子顺磁共振和机理研究证明,催化剂表面的分子氧转化为单线态氧 (1O2) 和超氧自由基阴离子 (•O2–)。1O2 的存在显着有助于 •O2– 的产生,从而有效促进乙苯的氧化。本研究介绍了一种新的活性氧 (ROS) 转化机制,并为碳氢化合物的选择性氧化提供了有价值的见解。
更新日期:2024-11-20
中文翻译:

La0.4Sr0.6CoO3催化的乙苯无溶剂选择性氧化制苯乙酮:一种由•O2–衍生的活性氧转化机制
开发一种绿色、稳定且具有成本效益的非均相催化剂,并阐明其在没有溶剂的情况下将 C-H 键选择性氧化成羰基化合物的催化机制具有重要的理论和实践价值。在此,我们使用溶胶-凝胶法合成钙钛矿催化剂,以催化乙苯的选择性氧化。值得注意的是,La0.4Sr0.6CoO 3-800(800 °C 是催化剂的煅烧温度)表现出显着的功效,将 73% 的乙苯转化为苯乙酮,选择性为 93%。表征分析表明,锶的掺入适度破坏了钙钛矿结构的内部平衡,导致氧空位增加和氧吸附能力增强。此外,电子顺磁共振和机理研究证明,催化剂表面的分子氧转化为单线态氧 (1O2) 和超氧自由基阴离子 (•O2–)。1O2 的存在显着有助于 •O2– 的产生,从而有效促进乙苯的氧化。本研究介绍了一种新的活性氧 (ROS) 转化机制,并为碳氢化合物的选择性氧化提供了有价值的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号