当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cellulose Nanocrystal-Immobilized Lipase for Pickering Interface Biocatalysis
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-19 , DOI: 10.1021/acssuschemeng.4c07051 Lili Li, Xiaojing Wang, Yali Hu, Wang Sun, Yugao Ding, Nisha He, Guofu Zhou, Zhen Zhang
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-19 , DOI: 10.1021/acssuschemeng.4c07051 Lili Li, Xiaojing Wang, Yali Hu, Wang Sun, Yugao Ding, Nisha He, Guofu Zhou, Zhen Zhang
|
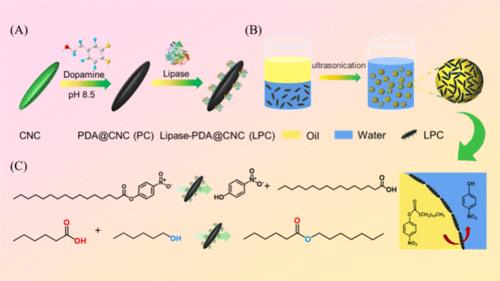
Enzymes are promising biocatalysts due to their high efficiency, mild conditions, high specificity, nontoxicity, and environmental friendliness. However, the enzyme suffers from poor stability and reusability. Enzyme immobilization is a commonly used technology to enhance its stability and reusability. In this study, the cellulose nanocrystal (CNC) was chosen as the enzyme immobilization carrier due to its sustainability, high specific surface area, biocompatibility, water dispersibility, and excellent Pickering emulsifying ability. Lipase was immobilized on a polydopamine-coated CNC (PC), and the obtained lipase-immobilized PC (LPC) displayed significantly enhanced stability and higher activity in harsh conditions compared to free lipase. LPC possessed partial wettability with both water and oil phases and a lower absolute value of the zeta potential than CNC, endowing it with a better Pickering emulsifying ability than CNC. LPC was fixed at the oil–water interface of the Pickering emulsion by ultrasonication, and the resulting Pickering emulsion droplets were employed as microreactors for ester hydrolysis and synthesis via Pickering interface biocatalysis. LPC displayed significantly enhanced catalysis activity at the oil–water interface of the Pickering emulsion compared to free lipase in a traditional oil–water biphasic system due to the increased contact area between the enzyme and the substrate, reduced diffusion distance of the substrate, and lipase interface activation effect. Moreover, LPC could be facilely recycled by centrifugation, and the recycled LPC still retains high catalysis activity. This study proposed a facile and sustainable method of enzyme immobilization to enhance its stability and reusability and achieved a significantly increased activity via Pickering interface biocatalysis.
中文翻译:

用于 Pickering 界面生物催化的纤维素纳米晶体固定化脂肪酶
酶因其高效、温和的条件、高特异性、无毒和环境友好性而成为有前途的生物催化剂。然而,该酶的稳定性和可重用性较差。酶固定化是一种常用的技术,可以提高其稳定性和可重用性。在本研究中,纤维素纳米晶体 (CNC) 因其可持续性、高比表面积、生物相容性、水分散性和优异的皮克林乳化能力而被选为酶固定载体。将脂肪酶固定在聚多巴胺包被的 CNC (PC) 上,与游离脂肪酶相比,获得的脂肪酶固定化 PC (LPC) 在恶劣条件下表现出显著增强的稳定性和更高的活性。LPC 在水相和油相中均具有部分润湿性,zeta 电位的绝对值低于 CNC,使其具有比 CNC 更好的皮克林乳化能力。通过超声将 LPC 固定在 Pickering 乳剂的油-水界面处,所得的 Pickering 乳化液滴被用作微反应器,通过 Pickering 界面生物催化进行酯水解和合成。与传统油水双相系统中的游离脂肪酶相比,LPC 在皮克林乳液的油-水界面处显示出显着增强的催化活性,这是由于酶与底物之间的接触面积增加,底物扩散距离减小,脂肪酶界面激活效应。此外,LPC 可以通过离心轻松回收,并且回收的 LPC 仍然保持较高的催化活性。 本研究提出了一种简单且可持续的酶固定化方法,以增强其稳定性和可重复使用性,并通过 Pickering 界面生物催化实现显着增加的活性。
更新日期:2024-11-20
中文翻译:

用于 Pickering 界面生物催化的纤维素纳米晶体固定化脂肪酶
酶因其高效、温和的条件、高特异性、无毒和环境友好性而成为有前途的生物催化剂。然而,该酶的稳定性和可重用性较差。酶固定化是一种常用的技术,可以提高其稳定性和可重用性。在本研究中,纤维素纳米晶体 (CNC) 因其可持续性、高比表面积、生物相容性、水分散性和优异的皮克林乳化能力而被选为酶固定载体。将脂肪酶固定在聚多巴胺包被的 CNC (PC) 上,与游离脂肪酶相比,获得的脂肪酶固定化 PC (LPC) 在恶劣条件下表现出显著增强的稳定性和更高的活性。LPC 在水相和油相中均具有部分润湿性,zeta 电位的绝对值低于 CNC,使其具有比 CNC 更好的皮克林乳化能力。通过超声将 LPC 固定在 Pickering 乳剂的油-水界面处,所得的 Pickering 乳化液滴被用作微反应器,通过 Pickering 界面生物催化进行酯水解和合成。与传统油水双相系统中的游离脂肪酶相比,LPC 在皮克林乳液的油-水界面处显示出显着增强的催化活性,这是由于酶与底物之间的接触面积增加,底物扩散距离减小,脂肪酶界面激活效应。此外,LPC 可以通过离心轻松回收,并且回收的 LPC 仍然保持较高的催化活性。 本研究提出了一种简单且可持续的酶固定化方法,以增强其稳定性和可重复使用性,并通过 Pickering 界面生物催化实现显着增加的活性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号