Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distinct Inflammatory Programs Underlie the Intramuscular Lipid Nanoparticle Response
ACS Nano ( IF 15.8 ) Pub Date : 2024-11-20 , DOI: 10.1021/acsnano.4c08490 William Dowell, Jacob Dearborn, Sylvester Languon, Zachary Miller, Tylar Kirch, Stephen Paige, Olivia Garvin, Lily Kjendal, Ethan Harby, Adam B. Zuchowski, Emily Clark, Carlos Lescieur-Garcia, Jesse Vix, Amy Schumer, Somen K. Mistri, Deena B. Snoke, Amber L. Doiron, Kalev Freeman, Michael J. Toth, Matthew E. Poynter, Jonathan E. Boyson, Devdoot Majumdar
ACS Nano ( IF 15.8 ) Pub Date : 2024-11-20 , DOI: 10.1021/acsnano.4c08490 William Dowell, Jacob Dearborn, Sylvester Languon, Zachary Miller, Tylar Kirch, Stephen Paige, Olivia Garvin, Lily Kjendal, Ethan Harby, Adam B. Zuchowski, Emily Clark, Carlos Lescieur-Garcia, Jesse Vix, Amy Schumer, Somen K. Mistri, Deena B. Snoke, Amber L. Doiron, Kalev Freeman, Michael J. Toth, Matthew E. Poynter, Jonathan E. Boyson, Devdoot Majumdar
|
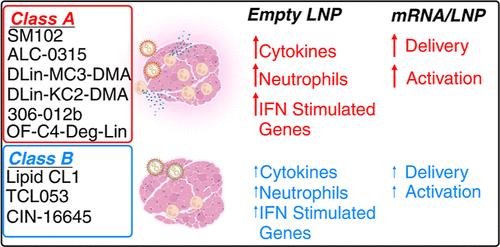
Developments in mRNA/lipid nanoparticle (LNP) technology have advanced the fields of vaccinology and gene therapy, raising questions about immunogenicity. While some mRNA/LNPs generate an adjuvant-like environment in muscle tissue, other mRNA/LNPs are distinct in their capacity for multiple rounds of therapeutic delivery. We evaluate the adjuvancy of components of mRNA/LNPs by phenotyping cellular infiltrate at injection sites, tracking uptake by immune cells, and assessing the inflammatory state. Delivery of 9 common, but chemically distinct, LNPs to muscle revealed two classes of inflammatory gene expression programs: inflammatory (Class A) and noninflammatory (Class B). We find that intramuscular injection with Class A, but not Class B, empty LNPs (eLNPs) induce robust neutrophil infiltration into muscle within 2 h and a diverse myeloid population within 24 h. Single-cell RNA sequencing revealed SM-102-mediated expression of inflammatory chemokines by myeloid infiltrates within muscle 1 day after injection. Surprisingly, we found direct transfection of muscle infiltrating myeloid cells and splenocytes 24 h after intramuscular mRNA/LNP administration. Transfected myeloid cells within the muscle exhibit an activated phenotype 24 h after injection. Similarly, directly transfected splenic lymphocytes and dendritic cells (DCs) are differentially activated by Class A or Class B containing mRNA/LNP. Within the splenic DC compartment, type II conventional DCs (cDC2s) are directly transfected and activated by Class A mRNA/LNP. Together, we show that mRNA and LNPs work synergistically to provide the necessary innate immune stimuli required for effective vaccination. Importantly, this work provides a design framework for vaccines and therapeutics alike.
中文翻译:

不同的炎症程序是肌内脂质纳米颗粒反应的基础
mRNA/脂质纳米颗粒 (LNP) 技术的发展推动了疫苗学和基因治疗领域的发展,引发了对免疫原性的问题。虽然一些 mRNA/LNP 在肌肉组织中产生类似佐剂的环境,但其他 mRNA/LNP 在多轮治疗递送的能力上是不同的。我们通过对注射部位的细胞浸润进行表型分析、跟踪免疫细胞的摄取和评估炎症状态来评估 mRNA/LNP 成分的佐剂。将 9 种常见但化学性质不同的 LNP 递送到肌肉揭示了两类炎症基因表达程序:炎症性(A 类)和非炎症性(B 类)。我们发现,用 A 类而不是 B 类空 LNP (eLNP) 肌肉注射在 2 小时内诱导强壮的中性粒细胞浸润到肌肉中,在 24 小时内诱导多样化的髓系群体。单细胞 RNA 测序显示注射后 1 天肌肉内髓系浸润介导的炎症趋化因子的 SM-102 介导表达。令人惊讶的是,我们发现肌肉注射 mRNA/LNP 给药后 24 小时肌肉浸润髓细胞和脾细胞的直接转染。注射后 24 小时,肌肉内转染的髓系细胞表现出活化的表型。同样,直接转染的脾淋巴细胞和树突状细胞 (DC) 被含有 mRNA/LNP 的 A 类或 B 类细胞差异激活。在脾 DC 区室内,II 型常规 DC (cDC2) 被 A 类 mRNA/LNP 直接转染和激活。我们共同表明,mRNA 和 LNP 协同作用,提供有效疫苗接种所需的必要先天免疫刺激。重要的是,这项工作为疫苗和治疗方法提供了一个设计框架。
更新日期:2024-11-20
中文翻译:

不同的炎症程序是肌内脂质纳米颗粒反应的基础
mRNA/脂质纳米颗粒 (LNP) 技术的发展推动了疫苗学和基因治疗领域的发展,引发了对免疫原性的问题。虽然一些 mRNA/LNP 在肌肉组织中产生类似佐剂的环境,但其他 mRNA/LNP 在多轮治疗递送的能力上是不同的。我们通过对注射部位的细胞浸润进行表型分析、跟踪免疫细胞的摄取和评估炎症状态来评估 mRNA/LNP 成分的佐剂。将 9 种常见但化学性质不同的 LNP 递送到肌肉揭示了两类炎症基因表达程序:炎症性(A 类)和非炎症性(B 类)。我们发现,用 A 类而不是 B 类空 LNP (eLNP) 肌肉注射在 2 小时内诱导强壮的中性粒细胞浸润到肌肉中,在 24 小时内诱导多样化的髓系群体。单细胞 RNA 测序显示注射后 1 天肌肉内髓系浸润介导的炎症趋化因子的 SM-102 介导表达。令人惊讶的是,我们发现肌肉注射 mRNA/LNP 给药后 24 小时肌肉浸润髓细胞和脾细胞的直接转染。注射后 24 小时,肌肉内转染的髓系细胞表现出活化的表型。同样,直接转染的脾淋巴细胞和树突状细胞 (DC) 被含有 mRNA/LNP 的 A 类或 B 类细胞差异激活。在脾 DC 区室内,II 型常规 DC (cDC2) 被 A 类 mRNA/LNP 直接转染和激活。我们共同表明,mRNA 和 LNP 协同作用,提供有效疫苗接种所需的必要先天免疫刺激。重要的是,这项工作为疫苗和治疗方法提供了一个设计框架。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号