当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unlocking the Full Redox Capability of Organic Charge‐Transfer Complex in High‐Loading Electrodes for Organic Rechargeable Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2024-11-20 , DOI: 10.1002/aenm.202404116 Sechan Lee, Jihyeon Kim, Jihyun Hong, Kisuk Kang
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2024-11-20 , DOI: 10.1002/aenm.202404116 Sechan Lee, Jihyeon Kim, Jihyun Hong, Kisuk Kang
|
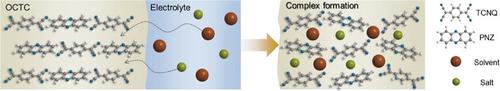
Organic charge‐transfer complex (OCTC) comprising redox‐active donor and acceptor molecules is a promising electrode material group, potentially resolving issues of low power and inferior cycle stability of organic electrodes in rechargeable batteries. Strong intermolecular interactions in OCTC such as π–π interaction and hydrogen bonding enable high electronic conductivity and suppress solubility to solvents. However, full redox activities of OCTC have not been achieved yet despite the inherent redox capabilities of respective donor and acceptor molecules. Here, it is revealed that the limited redox activities of OCTC stem from electrolyte‐incorporated complex formation, which weakens the characteristic intermolecular interactions, thereby hindering the redox reaction, particularly in Li‐based electrolytes. It is further shown that tailoring electrolyte types, specifically using Zn‐aqueous electrolytes, can substantially mitigate the complex formation and unlock the four‐electron redox activity of OCTC (phenazine (PNZ)‐7,7,8,8‐tetracyanoquinodimethane (TCNQ), that is, PNZ–TCNQ), with superior cycle stability retaining 88% of maximum capacity over 100 cycles. Surprisingly, the full redox reaction achieves an unprecedentedly high electrode‐level energy density, delivering ≈10 mAh cm−2 of areal capacity (580 µm‐thick electrodes) in Zn‐aqueous batteries. The findings elucidate the complex interplay between organic electrodes and electrolytes in the charge storage mechanism, highlighting the importance of electrolyte design in developing organic electrode materials.
中文翻译:

在有机可充电电池的高负载电极中释放有机电荷转移络合物的全部氧化还原能力
由氧化还原活性供体和受体分子组成的有机电荷转移复合物 (OCTC) 是一种很有前途的电极材料组,有望解决可充电电池中有机电极的低功耗和循环稳定性差的问题。OCTC 中强分子间相互作用(如 π-π 相互作用和氢键)可实现高电子导电性并抑制对溶剂的溶解度。然而,尽管各自的供体和受体分子具有固有的氧化还原能力,但 OCTC 的完全氧化还原活性尚未实现。本文揭示了 OCTC 的有限氧化还原活性源于电解质掺入的复合物形成,这削弱了特征性的分子间相互作用,从而阻碍了氧化还原反应,尤其是在锂基电解质中。进一步表明,定制电解质类型,特别是使用 Zn-水性电解质,可以显着减轻复合物的形成并释放 OCTC(吩嗪 (PNZ)‐7,7,8,8-四氰基喹代二甲烷 (TCNQ),即 PNZ-TCNQ),具有卓越的循环稳定性,可在 100 次循环中保持 88% 的最大容量。令人惊讶的是,全氧化还原反应实现了前所未有的高电极级能量密度,在锌水系电池中提供 ≈10 mAh cm-2 的面容量(580 μm 厚的电极)。研究结果阐明了有机电极和电解质在电荷存储机制中复杂的相互作用,突出了电解质设计在开发有机电极材料中的重要性。
更新日期:2024-11-20
中文翻译:

在有机可充电电池的高负载电极中释放有机电荷转移络合物的全部氧化还原能力
由氧化还原活性供体和受体分子组成的有机电荷转移复合物 (OCTC) 是一种很有前途的电极材料组,有望解决可充电电池中有机电极的低功耗和循环稳定性差的问题。OCTC 中强分子间相互作用(如 π-π 相互作用和氢键)可实现高电子导电性并抑制对溶剂的溶解度。然而,尽管各自的供体和受体分子具有固有的氧化还原能力,但 OCTC 的完全氧化还原活性尚未实现。本文揭示了 OCTC 的有限氧化还原活性源于电解质掺入的复合物形成,这削弱了特征性的分子间相互作用,从而阻碍了氧化还原反应,尤其是在锂基电解质中。进一步表明,定制电解质类型,特别是使用 Zn-水性电解质,可以显着减轻复合物的形成并释放 OCTC(吩嗪 (PNZ)‐7,7,8,8-四氰基喹代二甲烷 (TCNQ),即 PNZ-TCNQ),具有卓越的循环稳定性,可在 100 次循环中保持 88% 的最大容量。令人惊讶的是,全氧化还原反应实现了前所未有的高电极级能量密度,在锌水系电池中提供 ≈10 mAh cm-2 的面容量(580 μm 厚的电极)。研究结果阐明了有机电极和电解质在电荷存储机制中复杂的相互作用,突出了电解质设计在开发有机电极材料中的重要性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号