Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Probing of Dopamine Dynamics During Poly(I:C)-Induced Neuroinflammation
Small ( IF 13.0 ) Pub Date : 2024-11-20 , DOI: 10.1002/smll.202407685 Jian Wang, Qiang Zhou, Yuchan Zhang, Shuang Zhao, Li Li, Zhongyuan Zeng, Jiajia Chen, Yangmingxu Meng, Xianglong Zhao, Tianqi Wang, Zexuan Meng, Haihan Yuan, Jianhua Ran, Guixue Wang, Chen-zhong Li, Guangchao Zang
Small ( IF 13.0 ) Pub Date : 2024-11-20 , DOI: 10.1002/smll.202407685 Jian Wang, Qiang Zhou, Yuchan Zhang, Shuang Zhao, Li Li, Zhongyuan Zeng, Jiajia Chen, Yangmingxu Meng, Xianglong Zhao, Tianqi Wang, Zexuan Meng, Haihan Yuan, Jianhua Ran, Guixue Wang, Chen-zhong Li, Guangchao Zang
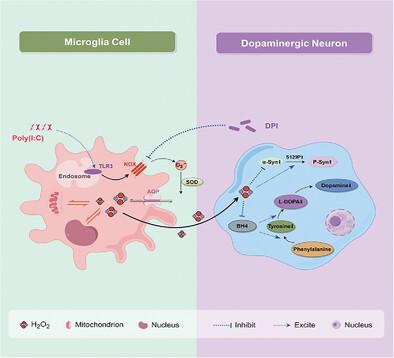
|
Viruses can infiltrate the central nervous system and contribute to depression, which may include alterations in dopamine (DA) metabolism triggered by immune responses though the specific mechanisms involved remain unclear. Here, an electrochemical system to realize the real-time dynamic monitoring of DA with high sensitivity is proposed and it is demonstrated that the viral simulator polyinosinic-polycytidylic acid (poly(I:C)) can inhibit the release of DA (from 5.595 to 0.137 µm) in neurons from the perspective of single cells, cell populations and even in vivo through the combination of multiscale electrodes, including single nanowires, carbon fibers (CFs) and 2D flexible electrodes. These findings are associated with the increase in reactive oxygen species (ROS) produced by microglia. At the molecular level, poly(I:C) significantly decreases the expression of α-synuclein and increases its phosphorylation level, whereas ROS inhibitors can reverse these pathological changes and salvage DA release to half the initial level (≈2.6 µM). These results suggest that viruses may indirectly inhibit DA system function through ROS produced in inflammatory responses and that antioxidant activity may be a potential therapeutic strategy.
中文翻译:

Poly(I:C) 诱导的神经炎症过程中多巴胺动力学的电化学探测
病毒可以渗透到中枢神经系统并导致抑郁症,这可能包括免疫反应触发的多巴胺 (DA) 代谢的改变,但所涉及的具体机制尚不清楚。在此,提出了一种实现高灵敏度 DA 实时动态监测的电化学系统,并证明了病毒模拟器聚肌苷-聚胞苷酸 (poly(I:C)) 可以通过多尺度电极的组合,从单细胞、细胞群甚至体内的角度抑制神经元中 DA 的释放(从 5.595 到 0.137 μm), 包括单纳米线、碳纤维 (CF) 和 2D 柔性电极。这些发现与小胶质细胞产生的活性氧 (ROS) 的增加有关。在分子水平上,poly(I:C) 显着降低 α-突触核蛋白的表达并增加其磷酸化水平,而 ROS 抑制剂可以逆转这些病理变化并将 DA 释放挽救至初始水平的一半 (≈2.6 μM)。这些结果表明,病毒可能通过炎症反应中产生的 ROS 间接抑制 DA 系统功能,并且抗氧化活性可能是一种潜在的治疗策略。
更新日期:2024-11-20
中文翻译:

Poly(I:C) 诱导的神经炎症过程中多巴胺动力学的电化学探测
病毒可以渗透到中枢神经系统并导致抑郁症,这可能包括免疫反应触发的多巴胺 (DA) 代谢的改变,但所涉及的具体机制尚不清楚。在此,提出了一种实现高灵敏度 DA 实时动态监测的电化学系统,并证明了病毒模拟器聚肌苷-聚胞苷酸 (poly(I:C)) 可以通过多尺度电极的组合,从单细胞、细胞群甚至体内的角度抑制神经元中 DA 的释放(从 5.595 到 0.137 μm), 包括单纳米线、碳纤维 (CF) 和 2D 柔性电极。这些发现与小胶质细胞产生的活性氧 (ROS) 的增加有关。在分子水平上,poly(I:C) 显着降低 α-突触核蛋白的表达并增加其磷酸化水平,而 ROS 抑制剂可以逆转这些病理变化并将 DA 释放挽救至初始水平的一半 (≈2.6 μM)。这些结果表明,病毒可能通过炎症反应中产生的 ROS 间接抑制 DA 系统功能,并且抗氧化活性可能是一种潜在的治疗策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号