当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Highly Fluorescent Thiazole Fused Benzo[a] Carbazoles by Sunlight Driven Photocyclization of Indolylthiazoles
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-11-20 , DOI: 10.1002/ejoc.202400972 Prabhas Bhaumick, Nurabul Mondal, Lokman H. Choudhury
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-11-20 , DOI: 10.1002/ejoc.202400972 Prabhas Bhaumick, Nurabul Mondal, Lokman H. Choudhury
|
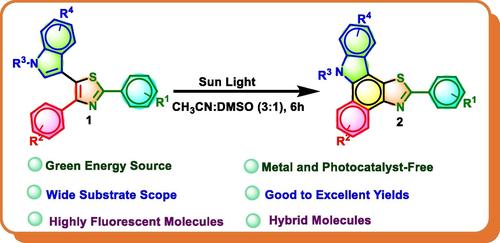
Herein we report for the first time a sunlight‐driven, irreversible photocyclization reaction of indole‐linked trisubstituted thiazoles, for the synthesis of highly fluorescent thiazole‐fused benzo[a ]carbazoles using a mixture of solvents (CH3 CN: DMSO; 3 : 1). Ring opening of indole moiety was observed in the case of thiazole derivatives having 2‐methyl indole substituents. Under similar reaction conditions, photocyclization of trisubstituted thiazoles having cyclic 1,3‐dicarbonyls in place of indole moiety also worked. This reaction provides products having two medicinally important moieties thiazole and benzocarbazoles. We have studied the photophysical properties of all the products and found that most of the synthesized products have very good fluorescence quantum yields.
中文翻译:

通过太阳光驱动吲哚基噻唑光环化合成高荧光噻唑熔融苯并[a] 咔唑
在此,我们首次报道了吲哚连接的三取代噻唑的阳光驱动的不可逆光环化反应,用于使用溶剂混合物合成高荧光噻唑熔融苯并[a]咔唑(CH3CN:DMSO;3:1)。在具有 2-甲基吲哚取代基的噻唑衍生物的情况下观察到吲哚部分的开环。在类似的反应条件下,具有环状 1,3-二羰基代替吲哚部分的三取代噻唑的光环化也有效。该反应提供具有两个具有重要药用部分的产物噻唑和苯并卡唑。我们研究了所有产物的光物理性质,发现大多数合成产物具有非常好的荧光量子产率。
更新日期:2024-11-20
中文翻译:

通过太阳光驱动吲哚基噻唑光环化合成高荧光噻唑熔融苯并[a] 咔唑
在此,我们首次报道了吲哚连接的三取代噻唑的阳光驱动的不可逆光环化反应,用于使用溶剂混合物合成高荧光噻唑熔融苯并[a]咔唑(CH3CN:DMSO;3:1)。在具有 2-甲基吲哚取代基的噻唑衍生物的情况下观察到吲哚部分的开环。在类似的反应条件下,具有环状 1,3-二羰基代替吲哚部分的三取代噻唑的光环化也有效。该反应提供具有两个具有重要药用部分的产物噻唑和苯并卡唑。我们研究了所有产物的光物理性质,发现大多数合成产物具有非常好的荧光量子产率。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号