Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrasound-Triggered NO Release to Promote Axonal Regeneration for Noise-Induced Hearing Loss Therapy
ACS Nano ( IF 15.8 ) Pub Date : 2024-11-19 , DOI: 10.1021/acsnano.4c12676 Binjun Chen, Yanhong Sun, Haojie Sun, Ning Cong, Rui Ma, Xiaoqing Qian, Jihan Lyu, Xiao Fu, Fanglu Chi, Hongzhe Li, Yanyan Liu, Dongdong Ren, Wenbo Bu
ACS Nano ( IF 15.8 ) Pub Date : 2024-11-19 , DOI: 10.1021/acsnano.4c12676 Binjun Chen, Yanhong Sun, Haojie Sun, Ning Cong, Rui Ma, Xiaoqing Qian, Jihan Lyu, Xiao Fu, Fanglu Chi, Hongzhe Li, Yanyan Liu, Dongdong Ren, Wenbo Bu
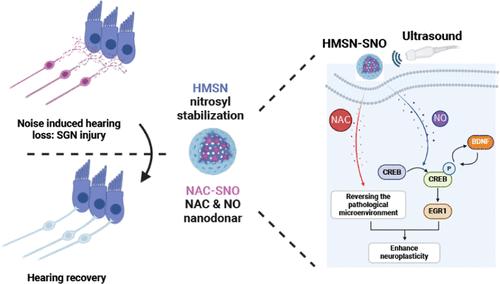
|
Intense noise poses a threat to spiral ganglion neurons (SGNs) in the inner ear, often resulting in limited axonal regeneration during noise injury and leading to noise-induced hearing loss (NIHL). Here, we propose an ultrasound-triggered nitric oxide (NO) release to enhance the sprouting and regeneration of injured axons in SGNs. We developed hollow silicon nanoparticles to load nitrosylated N-acetylcysteine, producing HMSN-SNO, which effectively protects NO from external interferences. Utilizing low-intensity ultrasound stimulation with bone penetration, we achieve the controlled release of NO from HMSN-SNO within the cochlea. In mice with NIHL, a rapid and extensive loss of synaptic connections between hair cells and SGNs is observed within 24 h after exposure to excessive noise. However, this loss could be reversed with the combined treatment, resulting in a hearing functional recovery from 83.57 to 65.00 dB SPL. This positive outcome is attributed to the multifunctional effects of HMSN-SNO, wherein they scavenge reactive oxygen species (ROS) to reverse the pathological microenvironment and simultaneously upregulate the CREB/BDNF/EGR1 signaling pathway, thereby enhancing neuroplasticity and promoting the regeneration of neuronal axons. These findings underscore the potential of nanomedicine for neuroplasticity modulation, which holds promise for advancing both basic research and the further treatment of neurological diseases.
中文翻译:

超声触发的 NO 释放促进轴突再生,用于噪声引起的听力损失治疗
强烈的噪声对内耳中的螺旋神经节神经元 (SGN) 构成威胁,通常会导致噪声损伤期间轴突再生受限,并导致噪声诱导性听力损失 (NIHL)。在这里,我们提出了一种超声触发的一氧化氮 (NO) 释放,以增强 SGN 中受伤轴突的发芽和再生。我们开发了空心硅纳米颗粒来负载亚硝基化 N-乙酰半胱氨酸,产生 HMSN-SNO,可有效保护 NO 免受外部干扰。利用具有骨穿透性的低强度超声刺激,我们实现了耳蜗内 HMSN-SNO 对 NO 的受控释放。在患有 NIHL 的小鼠中,在暴露于过度噪音后 24 小时内观察到毛细胞和 SGN 之间的突触连接快速而广泛地丧失。然而,这种损失可以通过联合治疗逆转,导致听力功能从 83.57 分贝恢复到 65.00 分贝 SPL。这一积极结果归因于 HMSN-SNO 的多功能效应,其中它们清除活性氧 (ROS) 以逆转病理微环境,同时上调 CREB/BDNF/EGR1 信号通路,从而增强神经可塑性并促进神经元轴突的再生。这些发现强调了纳米医学在神经可塑性调节方面的潜力,有望推进基础研究和神经系统疾病的进一步治疗。
更新日期:2024-11-20
中文翻译:

超声触发的 NO 释放促进轴突再生,用于噪声引起的听力损失治疗
强烈的噪声对内耳中的螺旋神经节神经元 (SGN) 构成威胁,通常会导致噪声损伤期间轴突再生受限,并导致噪声诱导性听力损失 (NIHL)。在这里,我们提出了一种超声触发的一氧化氮 (NO) 释放,以增强 SGN 中受伤轴突的发芽和再生。我们开发了空心硅纳米颗粒来负载亚硝基化 N-乙酰半胱氨酸,产生 HMSN-SNO,可有效保护 NO 免受外部干扰。利用具有骨穿透性的低强度超声刺激,我们实现了耳蜗内 HMSN-SNO 对 NO 的受控释放。在患有 NIHL 的小鼠中,在暴露于过度噪音后 24 小时内观察到毛细胞和 SGN 之间的突触连接快速而广泛地丧失。然而,这种损失可以通过联合治疗逆转,导致听力功能从 83.57 分贝恢复到 65.00 分贝 SPL。这一积极结果归因于 HMSN-SNO 的多功能效应,其中它们清除活性氧 (ROS) 以逆转病理微环境,同时上调 CREB/BDNF/EGR1 信号通路,从而增强神经可塑性并促进神经元轴突的再生。这些发现强调了纳米医学在神经可塑性调节方面的潜力,有望推进基础研究和神经系统疾病的进一步治疗。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号