当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peroxynitrite-Responsive Near-Infrared Fluorescent Imaging Guided Synergistic Chemo-Photodynamic Therapy via Biomimetic Metal–Organic Frameworks
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acsami.4c07389 Fangfang Yu, Tingya Wang, Yihan Wang, Liu Liu, Tengfei Liu, Wenyan Yao, Hongjie Xiong, Jiang Xiao, Xiaohui Liu, Hui Jiang, Xuemei Wang
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acsami.4c07389 Fangfang Yu, Tingya Wang, Yihan Wang, Liu Liu, Tengfei Liu, Wenyan Yao, Hongjie Xiong, Jiang Xiao, Xiaohui Liu, Hui Jiang, Xuemei Wang
|
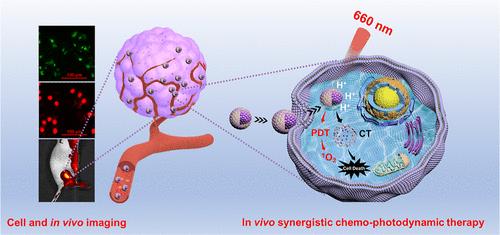
Peroxynitrite (ONOO–) plays a crucial role in maintaining cellular redox homeostasis and regulating diffusive processes, cellular transport, and signal transduction. Extensive studies have revealed that increased ONOO– levels during tumor progression are associated with heightened levels of oxidative stress. However, current methods lack noninvasive visualization, immediate reporting, and highly sensitive fluorescence sensing. In light of this, we have designed a biomimetic fluorescent nanoplatform, named Z-C-T@CM, for peroxynitrite-responsive near-infrared fluorescent imaging guided cancer treatment. The nanoplatform comprises tetrakis(4-carboxyphenyl) porphyrin (TCPP) and curcumin (CCM) encapsulated within a zeolitic imidazolate framework-8 (ZIF-8), which is coated with a mouse breast cancer cell membrane for enhanced biocompatibility and targeting, while evading immune clearance. In vitro experimental results demonstrate that the as-prepared nanoplatform exhibits enhanced near-infrared fluorescence emission upon exposure to ONOO–, indicating a significant potential for noninvasive in vivo imaging of ONOO– during tumor progression. Additionally, Z-C-T@CM readily degrades in the tumor microenvironment, releasing TCPP and CCM, enabling a synergistic chemo-photodynamic therapy with near-infrared illumination. Further investigations indicate that Z-C-T@CM efficiently stimulates a tumor immune response and facilitates therapeutic efficiency. Collectively, this work introduces a novel noninvasive strategy for ONOO– detection, shedding new light on the integration of cancer diagnosis and efficient treatment.
中文翻译:

过氧亚硝酸盐反应性近红外荧光成像通过仿生金属-有机框架引导协同化学-光动力疗法
过氧亚硝酸盐 (ONOO–) 在维持细胞氧化还原稳态和调节扩散过程、细胞转运和信号转导中起着至关重要的作用。广泛的研究表明,肿瘤进展过程中 ONOO– 水平升高与氧化应激水平升高有关。然而,目前的方法缺乏无创可视化、即时报告和高灵敏度荧光传感。有鉴于此,我们设计了一个名为 Z-C-T@CM 的仿生荧光纳米平台,用于过氧亚硝酸盐响应性近红外荧光成像引导癌症治疗。该纳米平台由四(4-羧基苯基)卟啉 (TCPP) 和姜黄素 (CCM) 组成,封装在沸石咪唑酸盐框架 8 (ZIF-8) 中,该框架涂有小鼠乳腺癌细胞膜以增强生物相容性和靶向性,同时逃避免疫清除。体外实验结果表明,所制备的纳米平台在暴露于 ONOO 时表现出增强的近红外荧光发射– 表明在肿瘤进展过程中对 ONOO – 进行无创体内成像具有显着的潜力。此外,Z-C-T@CM 在肿瘤微环境中容易降解,释放 TCPP 和 CCM,从而实现近红外照明的协同化学光动力疗法。进一步的研究表明,Z-C-T@CM 可有效刺激肿瘤免疫反应并促进治疗效率。总的来说,这项工作引入了一种新的无创 ONOO 检测策略,为癌症诊断和有效治疗的整合提供了新的思路。
更新日期:2024-11-19
中文翻译:

过氧亚硝酸盐反应性近红外荧光成像通过仿生金属-有机框架引导协同化学-光动力疗法
过氧亚硝酸盐 (ONOO–) 在维持细胞氧化还原稳态和调节扩散过程、细胞转运和信号转导中起着至关重要的作用。广泛的研究表明,肿瘤进展过程中 ONOO– 水平升高与氧化应激水平升高有关。然而,目前的方法缺乏无创可视化、即时报告和高灵敏度荧光传感。有鉴于此,我们设计了一个名为 Z-C-T@CM 的仿生荧光纳米平台,用于过氧亚硝酸盐响应性近红外荧光成像引导癌症治疗。该纳米平台由四(4-羧基苯基)卟啉 (TCPP) 和姜黄素 (CCM) 组成,封装在沸石咪唑酸盐框架 8 (ZIF-8) 中,该框架涂有小鼠乳腺癌细胞膜以增强生物相容性和靶向性,同时逃避免疫清除。体外实验结果表明,所制备的纳米平台在暴露于 ONOO 时表现出增强的近红外荧光发射– 表明在肿瘤进展过程中对 ONOO – 进行无创体内成像具有显着的潜力。此外,Z-C-T@CM 在肿瘤微环境中容易降解,释放 TCPP 和 CCM,从而实现近红外照明的协同化学光动力疗法。进一步的研究表明,Z-C-T@CM 可有效刺激肿瘤免疫反应并促进治疗效率。总的来说,这项工作引入了一种新的无创 ONOO 检测策略,为癌症诊断和有效治疗的整合提供了新的思路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号