当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Viologen–cycloparaphenylene hybrids: luminescent molecular nanocarbons for anion binding and specific vapor sorption
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-19 , DOI: 10.1039/d4qo01993h Rafał Frydrych, Kabali Senthilkumar, Katarzyna Ślusarek, Mateusz Waliczek, Wojciech Bury, Piotr J. Chmielewski, Joanna Cybińska, Marcin Stępień
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-19 , DOI: 10.1039/d4qo01993h Rafał Frydrych, Kabali Senthilkumar, Katarzyna Ślusarek, Mateusz Waliczek, Wojciech Bury, Piotr J. Chmielewski, Joanna Cybińska, Marcin Stępień
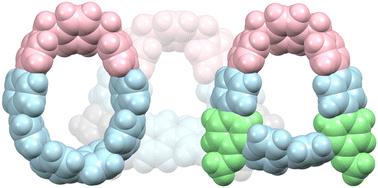
|
Two viologen-like macrocyclic receptors, [1]2+ and [2]2+, merging a bent oligophenylene loop with a (para-xylylene)bispyridinium moiety were designed and synthesized. Both dications were derived from the same precursor: the more strained cycloparaphenylene analogue [1]2+ was obtained using a reductive aromatization, whereas the less curved, meta-phenylene-containing [2]2+ was generated using a double eliminative rearrangement. [1]2+ is active as a receptor toward pyrene-1,3,6,8-tetrasulfonate, displaying a 2 : 1 binding interaction, with K11 = 7.4(3) × 103 M−1. In the solid state, [1][TFA]2 shows differential sorption of C6 hydrocarbons and shows reversible sorption of water. [1]2+ displays yellow emission in solution (λemmax = 544 nm in DMF), which is considerably red shifted in the solid state (λemmax = 644 nm for dry samples). Both [1]2+ and [2]2+ undergo electrochemical reduction, yielding transient viologen-like radical cations and diradicals.
中文翻译:

Viologen-cycloparaphenene 杂化物:用于阴离子结合和特异性蒸汽吸附的发光分子纳米碳
设计并合成了两个紫精样大环受体 [1]2+ 和 [2]2+,它们合并了一个弯曲的寡苯环和一个(对二甲苯)双吡啶部分。两种离子都来自相同的前体:更应变的环对苯类似物 [1]2+ 是使用还原芳构化获得的,而弯曲程度较低、含有间苯烯的 [2]2+ 是使用双消除重排产生的。[1]2+ 作为芘-1,3,6,8-四磺酸盐的受体具有活性,显示出 2 : 1 结合相互作用,K11 = 7.4(3) × 103 M-1。在固态下,[1][TFA]2 显示 C6 烃的差异吸附,并显示水的可逆吸附。[1]2+ 在溶液中显示黄色发射(DMF 中的 λemmax = 544 nm),在固态中红移很大(干燥样品的 λemmax = 644 nm)。[1]2+ 和 [2]2+ 都经历电化学还原,产生瞬态紫精样自由基阳离子和双自由基。
更新日期:2024-11-22
中文翻译:

Viologen-cycloparaphenene 杂化物:用于阴离子结合和特异性蒸汽吸附的发光分子纳米碳
设计并合成了两个紫精样大环受体 [1]2+ 和 [2]2+,它们合并了一个弯曲的寡苯环和一个(对二甲苯)双吡啶部分。两种离子都来自相同的前体:更应变的环对苯类似物 [1]2+ 是使用还原芳构化获得的,而弯曲程度较低、含有间苯烯的 [2]2+ 是使用双消除重排产生的。[1]2+ 作为芘-1,3,6,8-四磺酸盐的受体具有活性,显示出 2 : 1 结合相互作用,K11 = 7.4(3) × 103 M-1。在固态下,[1][TFA]2 显示 C6 烃的差异吸附,并显示水的可逆吸附。[1]2+ 在溶液中显示黄色发射(DMF 中的 λemmax = 544 nm),在固态中红移很大(干燥样品的 λemmax = 644 nm)。[1]2+ 和 [2]2+ 都经历电化学还原,产生瞬态紫精样自由基阳离子和双自由基。

































 京公网安备 11010802027423号
京公网安备 11010802027423号