当前位置:
X-MOL 学术
›
J. Mater. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and theoretical study on high hydrogen storage performance of Mg(NH2)2-2LiH composite system driven by nano CeO2 oxygen vacancies
Journal of Materials Science & Technology ( IF 11.2 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.jmst.2024.09.050 Haoyuan Zheng, Yuxiao Jia, Chen Jin, Hang Che, , Guang Liu, Li Wang, Yuyuan Zhao, Shixuan He, Haizhen Liu, Xinhua Wang, Yifeng Yu, Mi Yan
Journal of Materials Science & Technology ( IF 11.2 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.jmst.2024.09.050 Haoyuan Zheng, Yuxiao Jia, Chen Jin, Hang Che, , Guang Liu, Li Wang, Yuyuan Zhao, Shixuan He, Haizhen Liu, Xinhua Wang, Yifeng Yu, Mi Yan
|
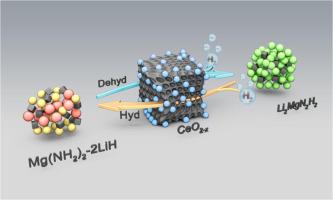
The magnesium based metal hydrogen storage composite system Mg(NH2)2-2LiH has a theoretical hydrogen storage capacity of 5.6 wt.% and is a promising hydrogen storage material for vehicles. However, its application is limited due to serious thermodynamic and kinetic barriers. Introducing efficient catalysts is an effective method to improve the hydrogen storage performance of Mg(NH2)2-2LiH. This article investigates for the first time the use of nano rare earth oxide CeO2 (∼44.5 nm) as an efficient modifier, achieving comprehensive regulation of the hydrogen storage performance of Mg(NH2)2-2LiH composite system through oxygen vacancy driven catalysis. The modification mechanism of nano CeO2 is also systematically studied using density functional theory (DFT) calculations and experimental results. Research has shown that the comprehensive hydrogen storage performance of the Mg(NH2)2-2LiH-5 wt.% CeO2 composite system is optimal, with high hydrogen absorption and desorption kinetics and reversible performance. The initial hydrogen absorption and desorption temperatures of the composite system were significantly reduced from 110/130°C to 65/80°C, and the release of by-product ammonia was significantly inhibited. Under the conditions of 170°C/50 min and 180°C/100 min, 4.37 wt.% of hydrogen can be rapidly absorbed and released. After 10 cycles of hydrogen release, the hydrogen cycle retention rate increased from 85% to nearly 100%. Further mechanistic studies have shown that the nano CeO2−x generated in situ during hydrogen evolution can effectively weaken the Mg–N and N–H bonds of Mg(NH2)2, exhibiting good catalytic effects. Meanwhile, oxygen vacancies provide a fast pathway for the diffusion of hydrogen atoms in the composite system. In addition, nano CeO2−x can effectively inhibit the polycrystalline transformation of the hydrogen evolving product Li2MgN2H2 in the system at high temperatures, reducing the difficulty of re-hydrogenation of the system. This study provides an innovative perspective for the efficient modification of magnesium based metal hydrogen storage composite materials using rare earth based catalysts, and also provides a reference for regulating the comprehensive hydrogen storage performance of hydrogen storage materials using rare earth catalysts with oxygen vacancies.
中文翻译:

纳米 CeO2 氧空位驱动 Mg(NH2)2-2LiH 复合体系高储氢性能的实验与理论研究
镁基金属储氢复合系统 Mg(NH2)2-2LiH 的理论储氢容量为 5.6 wt.%,是一种很有前途的车辆储氢材料。然而,由于严重的热力学和动力学障碍,其应用受到限制。引入高效催化剂是提高 Mg(NH2)2-2LiH 储氢性能的有效方法。本文首次研究了纳米稀土氧化物 CeO2 (∼44.5 nm) 作为高效改性剂的使用,通过氧空位驱动催化实现了对 Mg(NH2)2-2LiH 复合体系储氢性能的综合调控。还使用密度泛函理论 (DFT) 计算和实验结果系统研究了纳米 CeO2 的改性机理。研究表明,Mg(NH2)2-2LiH-5 wt.% CeO2 复合体系的综合储氢性能最佳,具有高吸氢和脱附动力学以及可逆性能。复合体系的初始吸氢和解吸温度从 110/130°C 显著降低到 65/80°C,副产物氨的释放得到显著抑制。在 170°C/50 min 和 180°C/100 min 的条件下,4.37 wt.% 的氢气可以被快速吸收和释放。氢释放 10 次循环后,氢循环保留率从 85% 提高到近 100%。 进一步的机理研究表明,析氢过程中原位生成的纳米 CeO2−x 可以有效削弱 Mg(NH2)2 的 Mg-N 和 N-H 键,表现出良好的催化作用。同时,氧空位为氢原子在复合系统中的扩散提供了快速途径。此外,纳米 CeO2−x 可以有效抑制析氢产物 Li2MgN2H2 在高温下体系中的多晶转变,降低体系再氢化的难度。本研究为利用稀土基催化剂对镁基金属储氢复合材料的高效改性提供了创新视角,也为利用含氧空位的稀土催化剂调控储氢材料的综合储氢性能提供了参考。
更新日期:2024-11-19
中文翻译:

纳米 CeO2 氧空位驱动 Mg(NH2)2-2LiH 复合体系高储氢性能的实验与理论研究
镁基金属储氢复合系统 Mg(NH2)2-2LiH 的理论储氢容量为 5.6 wt.%,是一种很有前途的车辆储氢材料。然而,由于严重的热力学和动力学障碍,其应用受到限制。引入高效催化剂是提高 Mg(NH2)2-2LiH 储氢性能的有效方法。本文首次研究了纳米稀土氧化物 CeO2 (∼44.5 nm) 作为高效改性剂的使用,通过氧空位驱动催化实现了对 Mg(NH2)2-2LiH 复合体系储氢性能的综合调控。还使用密度泛函理论 (DFT) 计算和实验结果系统研究了纳米 CeO2 的改性机理。研究表明,Mg(NH2)2-2LiH-5 wt.% CeO2 复合体系的综合储氢性能最佳,具有高吸氢和脱附动力学以及可逆性能。复合体系的初始吸氢和解吸温度从 110/130°C 显著降低到 65/80°C,副产物氨的释放得到显著抑制。在 170°C/50 min 和 180°C/100 min 的条件下,4.37 wt.% 的氢气可以被快速吸收和释放。氢释放 10 次循环后,氢循环保留率从 85% 提高到近 100%。 进一步的机理研究表明,析氢过程中原位生成的纳米 CeO2−x 可以有效削弱 Mg(NH2)2 的 Mg-N 和 N-H 键,表现出良好的催化作用。同时,氧空位为氢原子在复合系统中的扩散提供了快速途径。此外,纳米 CeO2−x 可以有效抑制析氢产物 Li2MgN2H2 在高温下体系中的多晶转变,降低体系再氢化的难度。本研究为利用稀土基催化剂对镁基金属储氢复合材料的高效改性提供了创新视角,也为利用含氧空位的稀土催化剂调控储氢材料的综合储氢性能提供了参考。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号