当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase conversion characteristics of lead sulfate in ferric sulfate medium
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.jhazmat.2024.136577 Pu Sun, Jibo Wang, Xingbin Li, Chang Wei, Zhigan Deng, Minting Li
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.jhazmat.2024.136577 Pu Sun, Jibo Wang, Xingbin Li, Chang Wei, Zhigan Deng, Minting Li
|
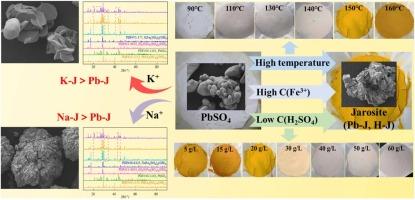
The phase transformation characteristics of lead sulfate (PbSO4) in a sulfuric acid (H2SO4) system were studied, focusing on the effects of temperature, H2SO4 concentration, ferric iron (Fe3+) concentration, sodium sulfate (Na2SO4), and potassium sulfate (K2SO4). The conversion of PbSO4 was analyzed by characterizing the composition and structure of the solid product through XRD, SEM/BSE-EDS, and FT-IR. The results indicated that the effect of temperature on PbSO4 transformation was influenced by H2SO4 concentration. The temperature threshold for PbSO4 conversion to lead jarosite (Pb-J) decreased from 150 to 90 ℃ as H2SO4 concentration decreased from 20 to 5 g/L. At 150 ℃, the amount of jarosite generated decreased significantly from 86.04 to 9.76 %. Subsequently, when H2SO4 concentration exceeded 40 g/L, PbSO4 was essentially unchanged. Concurrently, Pb-J formation correlated with the partial formation of hydronium jarosite (H-J). The production of jarosite was inhibited when the solution pH was ∼ 0.3 or lower. Furthermore, the increase in Fe3+ concentration facilitated Pb-J formation, whereas Na2SO4 and K2SO4 inhibited Pb-J formation, leading to the formation of potassium jarosite (K-J) and sodium jarosite (Na-J), respectively. This study provided insights into regulating PbSO4 conversion during sulfide ore oxygen pressure leaching.
中文翻译:

硫酸铁介质中硫酸铅的相转化特性
研究了硫酸铅 (PbSO4) 在硫酸 (H2SO4) 体系中的相变特性,重点研究了温度、H2SO4 浓度、三价铁 (Fe3+) 浓度、硫酸钠 (Na2SO4) 和硫酸钾 (K2SO4) 的影响。通过 XRD、SEM/BSE-EDS 和 FT-IR 表征固体产物的组成和结构,分析 PbSO4 的转化率。结果表明,温度对 PbSO4 转化的影响受 H2SO4 浓度的影响。当 H2SO4 浓度从 20 g/L 降至 5 g/L 时,PbSO4 转化为黄铁矿铅 (Pb-J) 的温度阈值从 150 °C 降至 90 °C。在 150 °C 时,黄铁矿的生成量从 86.04 % 显著下降到 9.76 %。随后,当 H2SO4 浓度超过 40 g/L 时,PbSO4 基本保持不变。同时,Pb-J 的形成与水合氢盐 (H-J) 的部分形成相关。当溶液 pH 值为 ∼ 0.3 或更低时,黄铁矿的产生受到抑制。此外,Fe3+ 浓度的增加促进了 Pb-J 的形成,而 Na2SO4 和 K2SO4 抑制了 Pb-J 的形成,分别导致黄铁矿钾 (K-J) 和黄铁矿钠 (Na-J) 的形成。本研究为硫化物矿石氧压浸出过程中调节 PbSO4 转化提供了见解。
更新日期:2024-11-20
中文翻译:

硫酸铁介质中硫酸铅的相转化特性
研究了硫酸铅 (PbSO4) 在硫酸 (H2SO4) 体系中的相变特性,重点研究了温度、H2SO4 浓度、三价铁 (Fe3+) 浓度、硫酸钠 (Na2SO4) 和硫酸钾 (K2SO4) 的影响。通过 XRD、SEM/BSE-EDS 和 FT-IR 表征固体产物的组成和结构,分析 PbSO4 的转化率。结果表明,温度对 PbSO4 转化的影响受 H2SO4 浓度的影响。当 H2SO4 浓度从 20 g/L 降至 5 g/L 时,PbSO4 转化为黄铁矿铅 (Pb-J) 的温度阈值从 150 °C 降至 90 °C。在 150 °C 时,黄铁矿的生成量从 86.04 % 显著下降到 9.76 %。随后,当 H2SO4 浓度超过 40 g/L 时,PbSO4 基本保持不变。同时,Pb-J 的形成与水合氢盐 (H-J) 的部分形成相关。当溶液 pH 值为 ∼ 0.3 或更低时,黄铁矿的产生受到抑制。此外,Fe3+ 浓度的增加促进了 Pb-J 的形成,而 Na2SO4 和 K2SO4 抑制了 Pb-J 的形成,分别导致黄铁矿钾 (K-J) 和黄铁矿钠 (Na-J) 的形成。本研究为硫化物矿石氧压浸出过程中调节 PbSO4 转化提供了见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号