当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protecting Group Control of Hydroxyketone-Hemiketal Tautomeric Equilibrium Enables the Stereoselective Synthesis of a 1′-Azido C-Nucleoside
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.joc.4c01981 Subhankar Panda, Moyosore O. Orimoloye, Tej Narayan Poudel, Steven De Jonghe, Dirk Jochmans, Johan Neyts, Courtney C. Aldrich
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.joc.4c01981 Subhankar Panda, Moyosore O. Orimoloye, Tej Narayan Poudel, Steven De Jonghe, Dirk Jochmans, Johan Neyts, Courtney C. Aldrich
|
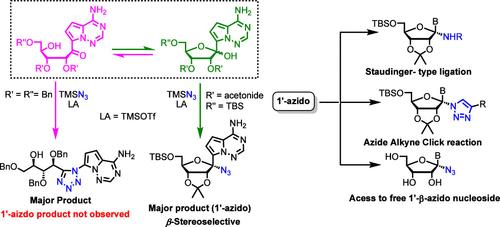
The synthesis of 1′-azido C-nucleosides is described to expand the set of azide-functionalized nucleosides for bioorthogonal applications and as potential antiviral drugs. Lewis acid-promoted azidation of a nucleoside hemiketal resulted in the formation of a tetrazole through a Schmidt reaction manifold. Conformational control to prevent ring–chain tautomerism enabled efficient 1′-azidation with complete β-diastereoselectivity. The unique reactivity and further derivation of the 1′-azido C-nucleosides are also reported.
中文翻译:

保护羟基酮-半基互变异构平衡的基团控制能够立体选择性合成 1′-叠氮基 C-核苷
1′-叠氮基 C-核苷的合成被描述为扩展叠氮化物官能化核苷的集合,用于生物正交应用和作为潜在的抗病毒药物。路易斯酸促进的核苷半脑胶氮化导致通过 Schmidt 反应歧管形成四唑。防止环链互变异构体的构象控制实现了高效的 1′-叠氮化和完全的 β-非对映选择性。还报道了 1′-叠氮基 C-核苷的独特反应性和进一步衍生。
更新日期:2024-11-19
中文翻译:

保护羟基酮-半基互变异构平衡的基团控制能够立体选择性合成 1′-叠氮基 C-核苷
1′-叠氮基 C-核苷的合成被描述为扩展叠氮化物官能化核苷的集合,用于生物正交应用和作为潜在的抗病毒药物。路易斯酸促进的核苷半脑胶氮化导致通过 Schmidt 反应歧管形成四唑。防止环链互变异构体的构象控制实现了高效的 1′-叠氮化和完全的 β-非对映选择性。还报道了 1′-叠氮基 C-核苷的独特反应性和进一步衍生。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号