当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
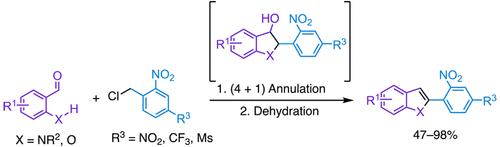
C2-Arylated Indoles and Benzofurans through Formal (4 + 1) Annulation of N-Sulfonyl-2-aminobenzaldehydes and Salicylaldehyde Derivatives with Electron-Deficient Benzyl Chlorides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.joc.4c02231 Lillian A. de Ceuninck van Capelle, Kimia Rahmannia, James M. Macdonald, Christopher Richardson, Michael G. Gardiner, John H. Ryan, Rasool Babaahmadi, Steven M. Wales, Christopher J. T. Hyland
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.joc.4c02231 Lillian A. de Ceuninck van Capelle, Kimia Rahmannia, James M. Macdonald, Christopher Richardson, Michael G. Gardiner, John H. Ryan, Rasool Babaahmadi, Steven M. Wales, Christopher J. T. Hyland
|
A two-step formal (4 + 1) annulation-dehydration reaction offers a convenient route to C2-arylated indoles and benzofurans. This reaction exploits the bifunctional reactivity of electron-deficient benzyl chlorides with N-sulfonyl-2-aminobenzaldehydes or salicylaldehyde derivatives. The reaction tolerates both electron-withdrawing and donating groups on the substituted aldehydes, as well as variation of electron-withdrawing groups at the para position of the benzyl chloride reagent. This work also identifies interesting byproducts resulting from the self-reaction of these benzyl chlorides under basic conditions.
中文翻译:

C2-芳基化吲哚和苯并呋喃通过与缺电子氯化苄的正式 (4 + 1) N-磺酰基-2-氨基苯甲醛和水杨醛衍生物的环化
两步法形式 (4 + 1) 环化-脱水反应为获得 C2-芳基化吲哚和苯并呋喃提供了一条便捷的途径。该反应利用了缺电子的氯化苄与 N-磺酰基-2-氨基苯甲醛或水杨醛衍生物的双功能反应性。该反应可容忍取代醛上的吸电子基团和供体基团,以及氯苄试剂对位处的吸电子基团的变化。这项工作还确定了这些氯化苄在碱性条件下自反应产生的有趣副产物。
更新日期:2024-11-19
中文翻译:

C2-芳基化吲哚和苯并呋喃通过与缺电子氯化苄的正式 (4 + 1) N-磺酰基-2-氨基苯甲醛和水杨醛衍生物的环化
两步法形式 (4 + 1) 环化-脱水反应为获得 C2-芳基化吲哚和苯并呋喃提供了一条便捷的途径。该反应利用了缺电子的氯化苄与 N-磺酰基-2-氨基苯甲醛或水杨醛衍生物的双功能反应性。该反应可容忍取代醛上的吸电子基团和供体基团,以及氯苄试剂对位处的吸电子基团的变化。这项工作还确定了这些氯化苄在碱性条件下自反应产生的有趣副产物。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号