当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Sulfinamidines via Iron-Catalyzed Nitrene Transfer Reaction with Sulfenamides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.joc.4c02286 Zhi-Kun Zhang, Yin Yuan, Huiling Peng, Yidan Han, Junliang Zhang, Junfeng Yang
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.joc.4c02286 Zhi-Kun Zhang, Yin Yuan, Huiling Peng, Yidan Han, Junliang Zhang, Junfeng Yang
|
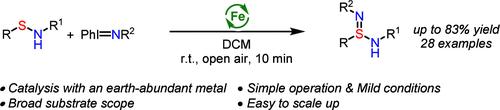
An iron-catalyzed nitrene transfer reaction for the rapid synthesis of sulfinamidines from readily available sulfenamides is reported. This method features mild conditions, short reaction times, and a broad substrate scope, allowing the preparation of a variety of sulfinamidines in good to excellent yields. The synthetic utility of the sulfinamidine products was further demonstrated through their conversion to other valuable sulfur(VI) compounds, such as sulfondiimidoyl fluorides, sulfinamidiate esters, and sulfonimidamides. Preliminary efforts in the development of an asymmetric variant showed moderate enantioselectivity.
中文翻译:

通过铁催化的硝酸酯与次磺酰胺转移反应合成磺胺脒
报道了一种铁催化的硝烯转移反应,用于从现成的次磺酰胺中快速合成磺胺脒。该方法条件温和、反应时间短、底物范围广,可以制备各种磺胺脒,产率从好到极好。通过将其转化为其他有价值的硫 (VI) 化合物,如磺酰二亚胺酰氟、亚磺酰胺酯和磺酰氨基酰胺,进一步证明了磺酰脒产品的合成效用。开发不对称变体的初步工作显示中等对映选择性。
更新日期:2024-11-19
中文翻译:

通过铁催化的硝酸酯与次磺酰胺转移反应合成磺胺脒
报道了一种铁催化的硝烯转移反应,用于从现成的次磺酰胺中快速合成磺胺脒。该方法条件温和、反应时间短、底物范围广,可以制备各种磺胺脒,产率从好到极好。通过将其转化为其他有价值的硫 (VI) 化合物,如磺酰二亚胺酰氟、亚磺酰胺酯和磺酰氨基酰胺,进一步证明了磺酰脒产品的合成效用。开发不对称变体的初步工作显示中等对映选择性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号