当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic EnT-Mediated Aminophosphorylation of Alkenes Using Oxime Esters as Bifunctional Reagents
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.orglett.4c03790 Xin Ruan, Di Wu, Chen Jiang, Cheng Chen, Yuhongxu Bai, Lin Tao, Caiyou Chen, Kai Wang, Xiang Li, Jun Jiang
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.orglett.4c03790 Xin Ruan, Di Wu, Chen Jiang, Cheng Chen, Yuhongxu Bai, Lin Tao, Caiyou Chen, Kai Wang, Xiang Li, Jun Jiang
|
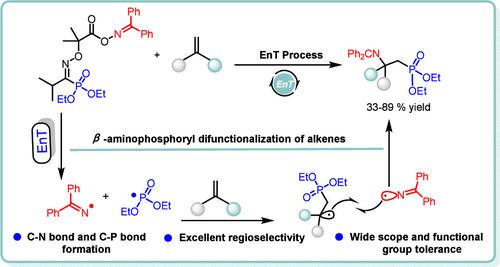
C–P bond formation has typically been achieved by a single-electron transfer process. Herein, a novel class of oxime ester bifunctionalization reagents were first applied to the photocatalytic β-aminophosphorylation of modular olefins. The bifunctional reagents generate two distinct radical species (imine and phosphoryl radicals) that exhibit excellent regioselectivity. Subsequently, these radicals are attached to the olefins through a single-step EnT catalytic process, establishing a novel synthetic pathway. This protocol is characterized by excellent regioselectivity, broad functional group tolerance, and mild reaction conditions, which would enrich the diversity and versatility to facilitate the diversity-oriented synthesis of β-aminophosphorylated complex molecule scaffolds.
中文翻译:

使用肟酯作为双功能试剂的光催化 EnT 介导的烯烃氨基磷酸化
C-P 键的形成通常是通过单电子转移过程实现的。在此,一类新型肟酯双功能化试剂首次应用于模块化烯烃的光催化 β-氨基磷酸化。双功能试剂可产生两种不同的自由基(亚胺和磷酸自由基),它们表现出优异的区域选择性。随后,这些自由基通过一步 EnT 催化过程连接到烯烃上,建立了一种新的合成途径。该方案的特点是出色的区域选择性、广泛的官能团耐受性和温和的反应条件,这将丰富多样性和多功能性,以促进 β-氨基磷酸化复合物分子支架的多样性合成。
更新日期:2024-11-19
中文翻译:

使用肟酯作为双功能试剂的光催化 EnT 介导的烯烃氨基磷酸化
C-P 键的形成通常是通过单电子转移过程实现的。在此,一类新型肟酯双功能化试剂首次应用于模块化烯烃的光催化 β-氨基磷酸化。双功能试剂可产生两种不同的自由基(亚胺和磷酸自由基),它们表现出优异的区域选择性。随后,这些自由基通过一步 EnT 催化过程连接到烯烃上,建立了一种新的合成途径。该方案的特点是出色的区域选择性、广泛的官能团耐受性和温和的反应条件,这将丰富多样性和多功能性,以促进 β-氨基磷酸化复合物分子支架的多样性合成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号