Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of Orange G on Activated Porous Carbon Derived from Coal Tar Pitch: Experimental and DFT Study
Langmuir ( IF 3.7 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.langmuir.4c03157 Linlin Huang, Xuwen Zhang, Tingting Liu, Lin Wang, Lixin Li, Da Li, Tao Sheng, Zilong Dong, Xinyue Zhao
Langmuir ( IF 3.7 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.langmuir.4c03157 Linlin Huang, Xuwen Zhang, Tingting Liu, Lin Wang, Lixin Li, Da Li, Tao Sheng, Zilong Dong, Xinyue Zhao
|
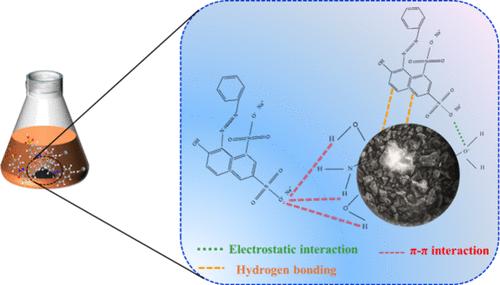
A coal tar pitch-based porous carbon adsorbent (CPA) was synthesized through a straightforward method involving the heating of a mixture of KOH and coal tar pitch (CTP). This CPA exhibited a high surface area of 1811.2 m2 g–1 and a large pore volume of 0.94 cm3 g–1 when prepared with a CTP to KOH mass ratio of 1:4 at 800 °C. Parameters such as the heating temperature and activator dose were optimized to enhance the adsorption efficiency. The prepared CPA was extensively characterized by SEM, XRD, FTIR, and BET measurements. Notably, CPA presented a distinct adsorption performance for Orange G (OG), achieving a maximum adsorption capability of 449.7 mg g–1. Kinetic studies indicated that the adsorption process followed the pseudo-second-order model, while the adsorption isotherm data demonstrated that both chemical and physical interactions favored OG adsorption. Thermodynamic analysis revealed that the adsorption of OG on CPA was spontaneous and exothermic and increased the entropy. Density functional theory (DFT) calculations provided insights into the adsorption mechanism, highlighting electrostatic interactions, hydrogen bonds, and π–π interactions as the dominant processes governing OG adsorption onto the adsorbent.
中文翻译:

橙 G 对煤焦油沥青活性多孔炭的吸附:实验和 DFT 研究
通过一种简单的方法合成了一种基于煤焦油沥青的多孔碳吸附剂 (CPA),该方法涉及加热 KOH 和煤焦油沥青 (CTP) 的混合物。在 800 °C 下以 1:4 的 CTP 与 KOH 质量比制备时,该 CPA 表现出 1811.2 m2 g–1 的高表面积和 0.94 cm3 g–1 的大孔体积。 优化加热温度和活化剂剂量等参数,以提高吸附效率。通过 SEM、XRD、FTIR 和 BET 测量对制备的 CPA 进行了广泛的表征。值得注意的是,CPA 对 Orange G (OG) 表现出独特的吸附性能,最大吸附能力为 449.7 mg g–1。动力学研究表明,吸附过程遵循准二级模型,而吸附等温线数据表明,化学和物理相互作用都有利于 OG 吸附。热力学分析表明,OG 对 CPA 的吸附是自发的和放热的,并且熵增加。密度泛函理论 (DFT) 计算提供了对吸附机制的见解,突出了静电相互作用、氢键和 π-π 相互作用是控制 OG 吸附到吸附剂上的主要过程。
更新日期:2024-11-19
中文翻译:

橙 G 对煤焦油沥青活性多孔炭的吸附:实验和 DFT 研究
通过一种简单的方法合成了一种基于煤焦油沥青的多孔碳吸附剂 (CPA),该方法涉及加热 KOH 和煤焦油沥青 (CTP) 的混合物。在 800 °C 下以 1:4 的 CTP 与 KOH 质量比制备时,该 CPA 表现出 1811.2 m2 g–1 的高表面积和 0.94 cm3 g–1 的大孔体积。 优化加热温度和活化剂剂量等参数,以提高吸附效率。通过 SEM、XRD、FTIR 和 BET 测量对制备的 CPA 进行了广泛的表征。值得注意的是,CPA 对 Orange G (OG) 表现出独特的吸附性能,最大吸附能力为 449.7 mg g–1。动力学研究表明,吸附过程遵循准二级模型,而吸附等温线数据表明,化学和物理相互作用都有利于 OG 吸附。热力学分析表明,OG 对 CPA 的吸附是自发的和放热的,并且熵增加。密度泛函理论 (DFT) 计算提供了对吸附机制的见解,突出了静电相互作用、氢键和 π-π 相互作用是控制 OG 吸附到吸附剂上的主要过程。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号