当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carboxylic acid-assisted hydration for the preparation of mesoporous magnesium hydroxide/magnesium oxide with ultra-high adsorption performance and selective adsorption: Experiments and DFT investigations
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.apsusc.2024.161849 Jun Li, Yanran Li, Ziyu Zhang, Rongzheng Gao, Zihan Zhao, Shichen Xing, Dongmo Wu, Heqi Qi, Dong Zhang, Xiaoli Tian, Cheng Liu, Lingling Zhu, Chengliang Ma
Applied Surface Science ( IF 6.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.apsusc.2024.161849 Jun Li, Yanran Li, Ziyu Zhang, Rongzheng Gao, Zihan Zhao, Shichen Xing, Dongmo Wu, Heqi Qi, Dong Zhang, Xiaoli Tian, Cheng Liu, Lingling Zhu, Chengliang Ma
|
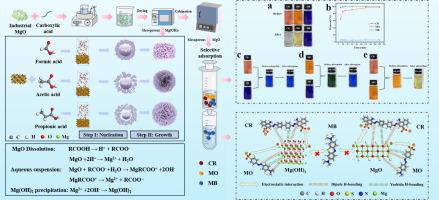
Herein, mesoporous Mg(OH)2 is prepared by one-step hydration method using microcrystalline magnesite as raw material and acetic acid as hydration agent, and the mesoporous MgO is obtained after thermal conversion. The adsorption experiments on three single dye systems (Congo red (CR), methyl orange (MO) and methylene blue (MB)) and three binary mixed dye systems (CR/MO, CR/MB and MO/MB) show that mesoporous MgO possesses superior adsorption selectivity towards anionic dyes. The mesoporous Mg(OH)2 with cardroom-like structure, obvious pore channel and abundant edge and concern shows good adsorption capacity for CR (6591.96 mg/g) and MO (3087.04 mg/g). The mesoporous MgO obtained by calcination has high specific surface area (66 m2/g) and partially inherits the cardroom-like structure of Mg(OH)2, exhibiting remarkable adsorption performance (CR: 10521.89 mg/g; MO: 6694.65 mg/g). CR and MO adsorption on the Mg(OH)2/MgO accords with the Langmuir model and pseudo-second-order kinetic model. The density functional theory (DFT) is employed to evaluate the reactivity of CR/MO, such as molecular frontier orbital energies and energy gap, demonstrating that CR exhibits superior reactivity and adsorptive capacity in comparison to MO. The MgO sample is regenerated by calcination after CR/MO adsorption and the CR removal efficiency of MgO reaches above 85 % after 10 cycles.
中文翻译:

羧酸辅助水合制备具有超高吸附性能和选择性吸附的介孔氢氧化镁/氧化镁:实验和 DFT 研究
本文以微晶菱镁矿为原料,以乙酸为水化剂,通过一步水化法制备介孔Mg(OH)2,热转化后得到介孔MgO。在三种单一染料体系(刚果红 (CR)、甲基橙 (MO) 和亚甲基蓝 (MB))和三种二元混合染料体系 (CR/MO、CR/MB 和 MO/MB) 上的吸附实验表明,介孔 MgO 对阴离子染料具有优异的吸附选择性。介孔 Mg(OH)2 具有药房状结构、明显的孔通道和丰富的边缘和关注度,对 CR (6591.96 mg/g) 和 MO (3087.04 mg/g) 具有良好的吸附能力。煅烧得到的介孔 MgO 具有高比表面积 (66 m2/g),部分继承了 Mg(OH)2 的棋盘状结构,表现出显著的吸附性能 (CR: 10521.89 mg/g;MO:6694.65 毫克/克)。CR 和 MO 在 Mg(OH)2/MgO 上的吸附符合 Langmuir 模型和准二级动力学模型。密度泛函理论 (DFT) 用于评估 CR/MO 的反应性,例如分子前沿轨道能量和能隙,证明 CR 与 MO 相比表现出优异的反应性和吸附能力。MgO 样品经 CR/MO 吸附后通过煅烧再生,循环 10 次后 MgO 的 CR 去除效率达到 85 % 以上。
更新日期:2024-11-19
中文翻译:

羧酸辅助水合制备具有超高吸附性能和选择性吸附的介孔氢氧化镁/氧化镁:实验和 DFT 研究
本文以微晶菱镁矿为原料,以乙酸为水化剂,通过一步水化法制备介孔Mg(OH)2,热转化后得到介孔MgO。在三种单一染料体系(刚果红 (CR)、甲基橙 (MO) 和亚甲基蓝 (MB))和三种二元混合染料体系 (CR/MO、CR/MB 和 MO/MB) 上的吸附实验表明,介孔 MgO 对阴离子染料具有优异的吸附选择性。介孔 Mg(OH)2 具有药房状结构、明显的孔通道和丰富的边缘和关注度,对 CR (6591.96 mg/g) 和 MO (3087.04 mg/g) 具有良好的吸附能力。煅烧得到的介孔 MgO 具有高比表面积 (66 m2/g),部分继承了 Mg(OH)2 的棋盘状结构,表现出显著的吸附性能 (CR: 10521.89 mg/g;MO:6694.65 毫克/克)。CR 和 MO 在 Mg(OH)2/MgO 上的吸附符合 Langmuir 模型和准二级动力学模型。密度泛函理论 (DFT) 用于评估 CR/MO 的反应性,例如分子前沿轨道能量和能隙,证明 CR 与 MO 相比表现出优异的反应性和吸附能力。MgO 样品经 CR/MO 吸附后通过煅烧再生,循环 10 次后 MgO 的 CR 去除效率达到 85 % 以上。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号