当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the synergistic catalysis of balanced Cu0-Cu+ sites and oxygen vacancies in Cu/ZrO2 catalysts for the efficient hydrogenation of furfural
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.cej.2024.157796 Xinling Yang, Zhou Chen, Jingjing Tan, Yuanna Zhang, Jinglei Cui, Changzhen Wang, Li Fang, Yulei Zhu, Long Huang, Hu Shi, Yongzhao Wang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.cej.2024.157796 Xinling Yang, Zhou Chen, Jingjing Tan, Yuanna Zhang, Jinglei Cui, Changzhen Wang, Li Fang, Yulei Zhu, Long Huang, Hu Shi, Yongzhao Wang
|
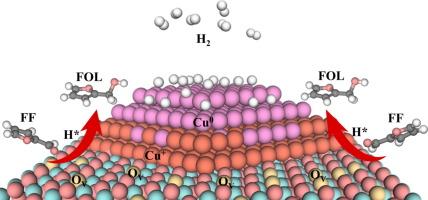
Identifying the active sites and synergistic catalytic effect for Cu-based catalysts remains challenging due to the evolving structures of Cu0 and Cu+ species during the hydrogenation reaction process. In this study, Cu/t-ZrO2 catalysts prepared by the oxalic acid complex precipitation method were explored for the hydrogenation of furfural to understand the catalytic functions of Cu0 and Cu+ during the reaction. The catalyst calcined at 300 °C (Cu/t-ZrO2 -300) exhibited a superior catalytic efficiency in furfural hydrogenation to furfuryl alcohol. A 100 % yield of furfuryl alcohol together with a high TOF of 24.2 h−1 was achieved at 120 °C. Extensive characterization, including in-situ FTIR, in-situ XPS, EPR, and Raman, substantiated that the excellent catalytic performance of the catalysts was assigned to the synergistic catalysis Cu+ , Cu0 and oxygen vacancies. Among them, Cu+ and oxygen vacancies in the catalyst were favorable to the adsorption and activation of the carbonyl group, where Cu+ sites were more preferred to adsorb CO and formed a stable complex compared to Cu0 active sites. Furthermore, the DFT calculations verified that these abundant oxygen vacancies in the Cu-ZrO2 interface induced a reverse of charge transfer from Zr to Cu atoms in the catalyst, resulting in a downshift of the d -band center for Cu, which was beneficial to the adsorption and activation of furfural and the desorption of active H*. Meanwhile, the results corroborated that more oxygen vacancies in the catalyst can regulate the adsorption configuration of furfural on the catalyst surface. This study elucidated the catalytic mechanism of complex active sites in Cu-based catalysts for furfural hydrogenation, and which will offer a valuable reference for the design of efficient catalysts for biomass platform conversion.
中文翻译:

了解 Cu/ZrO2 催化剂中平衡 Cu0-Cu+ 位点和氧空位的协同催化作用,用于糠醛的高效加氢
由于加氢反应过程中 Cu0 和 Cu+ 物质的结构不断演变,确定 Cu 基催化剂的活性位点和协同催化作用仍然具有挑战性。本研究探索了草酸络合沉淀法制备的 Cu/t-ZrO2 催化剂用于糠醛的加氢反应,以了解 Cu0 和 Cu+ 在反应过程中的催化功能。在 300 °C 下煅烧的催化剂 (Cu/t-ZrO2-300) 在糠醛加氢制糠醇中表现出优异的催化效率。在 120 °C 下,糠醇的收率为 100%,并且 TOF 高达 24.2 h-1。 广泛的表征,包括原位 FTIR、原位 XPS、EPR 和拉曼,证实了催化剂的出色催化性能被分配给协同催化 Cu+、Cu0 和氧空位。其中,催化剂中的 Cu+ 和氧空位有利于羰基的吸附和活化,其中 Cu+ 位点比吸附 CO 更有利,与 Cu0 活性位点相比,形成稳定的络合物。此外,DFT 计算验证了 Cu-ZrO2 界面中这些丰富的氧空位诱导催化剂中从 Zr 到 Cu 原子的电荷转移发生逆转,导致 Cu 的 d 带中心下移,这有利于糠醛的吸附和活化以及活性 H* 的解吸。同时,结果证实催化剂中更多的氧空位可以调节糠醛在催化剂表面的吸附构型。 本研究阐明了铜基催化剂中复杂活性位点对糠醛加氢的催化机制,将为设计用于生物质平台转化的高效催化剂提供有价值的参考。
更新日期:2024-11-19
中文翻译:

了解 Cu/ZrO2 催化剂中平衡 Cu0-Cu+ 位点和氧空位的协同催化作用,用于糠醛的高效加氢
由于加氢反应过程中 Cu0 和 Cu+ 物质的结构不断演变,确定 Cu 基催化剂的活性位点和协同催化作用仍然具有挑战性。本研究探索了草酸络合沉淀法制备的 Cu/t-ZrO2 催化剂用于糠醛的加氢反应,以了解 Cu0 和 Cu+ 在反应过程中的催化功能。在 300 °C 下煅烧的催化剂 (Cu/t-ZrO2-300) 在糠醛加氢制糠醇中表现出优异的催化效率。在 120 °C 下,糠醇的收率为 100%,并且 TOF 高达 24.2 h-1。 广泛的表征,包括原位 FTIR、原位 XPS、EPR 和拉曼,证实了催化剂的出色催化性能被分配给协同催化 Cu+、Cu0 和氧空位。其中,催化剂中的 Cu+ 和氧空位有利于羰基的吸附和活化,其中 Cu+ 位点比吸附 CO 更有利,与 Cu0 活性位点相比,形成稳定的络合物。此外,DFT 计算验证了 Cu-ZrO2 界面中这些丰富的氧空位诱导催化剂中从 Zr 到 Cu 原子的电荷转移发生逆转,导致 Cu 的 d 带中心下移,这有利于糠醛的吸附和活化以及活性 H* 的解吸。同时,结果证实催化剂中更多的氧空位可以调节糠醛在催化剂表面的吸附构型。 本研究阐明了铜基催化剂中复杂活性位点对糠醛加氢的催化机制,将为设计用于生物质平台转化的高效催化剂提供有价值的参考。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号