当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
5-Year Results From the AMPLATZER Amulet Left Atrial Appendage Occluder Randomized Controlled Trial
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.jacc.2024.10.101 Dhanunjaya Lakkireddy, Christopher R. Ellis, David Thaler, Vijendra Swarup, Alok Gambhir, James Hermiller, Jens Erik Nielsen-Kudsk, Stephen Worthley, Devi Nair, Boris Schmidt, Rodney Horton, Nigel Gupta, Jordan A. Anderson, Hong Zhao, Mohamad Alkhouli, Stephan Windecker
中文翻译:

AMPLATZER Amulet 左心耳封堵器随机对照试验的 5 年结果
Amulet IDE 试验(AMPLATZER Amulet 左心耳封堵器 [LAAO] 研究装置豁免 [IDE] 试验)评估了 Amulet 封堵器 (Abbott) 在非瓣膜性心房颤动患者中的安全性和有效性。Amulet IDE 试验是最大的随机 LAAO 试验,将 Amulet 封堵器与 Watchman 2.5 设备(波士顿科学公司)进行了比较。
本分析展示了 5 年试验的结果,将 2 种设备头对头进行比较。
参加 Amulet IDE 试验的患者发生中风或全身性栓塞的风险较高,定义为 CHADS2 评分 ≥2 或 CHA2DS2-VASc 评分 ≥3。口服抗凝药 (OAC) 的使用和主要临床结局在 5 年内呈报。
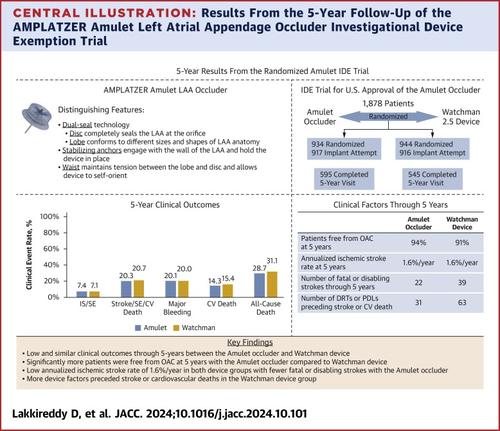
共有 1,878 名患者被随机分配,其中 1,833 名接受了设备植入尝试(n = 917,Amulet 封堵器;n = 916,Watchman 设备)。在每次随访时,Amulet 封堵器组无 OAC 的患者百分比显着更高,Amulet 和 Watchman 设备组在最近 5 年随访中分别有 94.0% 和 90.9% 的患者无 OAC (P = 0.009)。Amulet 和 Watchman 装置的 5 年临床结局相似,包括缺血性中风或全身性栓塞的复合 (7.4% vs 7.1%;P = 0.851)、中风、全身性栓塞或心血管死亡的复合 (20.3% 对 20.7%;P = 0.666)、大出血 (20.1% 对 20.0%;P = 0.882)、心血管 (CV) 死亡 (14.3% 对 15.4%;P = 0.429)和全因死亡 (28.7% 对 31.1%;P = 0.217)。5 年时年化缺血性卒中发生率较低,Amulet (1.6%/y) 和 Watchman (1.6%/y) 装置相同。与使用 Watchman 装置的患者相比,使用 Amulet 封堵器的患者中风更轻(n = 38,非致残;n = 11,致残;n = 11,致死;n = 12,未知)。此外,与使用 Amulet 封堵器的患者 (n = 31) 相比,使用 Watchman 装置的患者 (n = 63) 的设备因素 (设备相关血栓或设备周围泄漏 ≥ 3 mm) 更频繁地发生中风事件和 CV 死亡。
最大的随机 LAAO 临床试验的 5 年结果证明了 Amulet 封堵器和 Watchman 2.5 设备的长期安全性和有效性。双密封 Amulet 封堵器可减少与心房颤动相关的血栓栓塞事件,同时无需长期 OAC。(AMPLATZER Amulet 左心耳封堵器 [LAAO] 研究装置豁免 [IDE] 试验 [Amulet IDE 试验];NCT02879448)
更新日期:2024-11-19
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.jacc.2024.10.101 Dhanunjaya Lakkireddy, Christopher R. Ellis, David Thaler, Vijendra Swarup, Alok Gambhir, James Hermiller, Jens Erik Nielsen-Kudsk, Stephen Worthley, Devi Nair, Boris Schmidt, Rodney Horton, Nigel Gupta, Jordan A. Anderson, Hong Zhao, Mohamad Alkhouli, Stephan Windecker
|
Background
The Amulet IDE trial (AMPLATZER Amulet Left Atrial Appendage Occluder [LAAO] Investigational Device Exemption [IDE] Trial) evaluated the safety and effectiveness of the Amulet occluder (Abbott) in patients with nonvalvular atrial fibrillation. The Amulet IDE trial is the largest randomized LAAO trial, comparing the Amulet occluder with the Watchman 2.5 device (Boston Scientific).Objectives
This analysis presents the 5-year results from the trial comparing the 2 devices head to head.Methods
Patients enrolled in the Amulet IDE trial were at a high risk of stroke or systemic embolism defined as a CHADS2 score ≥2 or CHA2DS2-VASc score ≥3. Oral anticoagulation (OAC) use and key clinical outcomes are presented through 5 years.Results
A total of 1,878 patients were randomized, with 1,833 undergoing a device implantation attempt (n = 917, Amulet occluder; and n = 916, Watchman device). A significantly higher percentage of patients were free of OAC in the Amulet occluder group at each follow-up visit, with 94.0% and 90.9% free of OAC at the last 5-year follow-up visit in the Amulet and Watchman device groups, respectively (P = 0.009). The 5-year clinical outcomes were similar between the Amulet and Watchman devices, including the composite of ischemic stroke or systemic embolism (7.4% vs 7.1%; P = 0.851), the composite of stroke, systemic embolism, or cardiovascular death (20.3% vs 20.7%; P = 0.666), major bleeding (20.1% vs 20.0%; P = 0.882), cardiovascular (CV) death (14.3% vs 15.4%; P = 0.429), and all-cause death (28.7% vs 31.1%; P = 0.217). Annualized ischemic stroke rates at 5 years were low and the same for Amulet (1.6%/y) and Watchman (1.6%/y) devices. Strokes in patients with the Amulet occluder were less severe (n = 38, nondisabling; n = 11, disabling; n = 11, fatal; n = 12, unknown) than strokes in patients with the Watchman device (n = 19, nondisabling; n = 22, disabling; n = 17, fatal; n = 10, unknown). Moreover, device factors (device-related thrombus or peridevice leak ≥3 mm) preceded stroke events and CV deaths more frequently in patients with the Watchman device (n = 63) compared with patients with the Amulet occluder (n = 31).Conclusions
The 5-year outcomes from the largest randomized LAAO clinical trial demonstrated the long-term safety and effectiveness of the Amulet occluder and Watchman 2.5 devices. The dual-seal Amulet occluder reduces atrial fibrillation–related thromboembolic events while eliminating the need for long-term OAC. (AMPLATZER Amulet Left Atrial Appendage Occluder [LAAO] Investigational Device Exemption [IDE] Trial [Amulet IDE trial]; NCT02879448)中文翻译:

AMPLATZER Amulet 左心耳封堵器随机对照试验的 5 年结果
背景
Amulet IDE 试验(AMPLATZER Amulet 左心耳封堵器 [LAAO] 研究装置豁免 [IDE] 试验)评估了 Amulet 封堵器 (Abbott) 在非瓣膜性心房颤动患者中的安全性和有效性。Amulet IDE 试验是最大的随机 LAAO 试验,将 Amulet 封堵器与 Watchman 2.5 设备(波士顿科学公司)进行了比较。
目标
本分析展示了 5 年试验的结果,将 2 种设备头对头进行比较。
方法
参加 Amulet IDE 试验的患者发生中风或全身性栓塞的风险较高,定义为 CHADS2 评分 ≥2 或 CHA2DS2-VASc 评分 ≥3。口服抗凝药 (OAC) 的使用和主要临床结局在 5 年内呈报。
结果
共有 1,878 名患者被随机分配,其中 1,833 名接受了设备植入尝试(n = 917,Amulet 封堵器;n = 916,Watchman 设备)。在每次随访时,Amulet 封堵器组无 OAC 的患者百分比显着更高,Amulet 和 Watchman 设备组在最近 5 年随访中分别有 94.0% 和 90.9% 的患者无 OAC (P = 0.009)。Amulet 和 Watchman 装置的 5 年临床结局相似,包括缺血性中风或全身性栓塞的复合 (7.4% vs 7.1%;P = 0.851)、中风、全身性栓塞或心血管死亡的复合 (20.3% 对 20.7%;P = 0.666)、大出血 (20.1% 对 20.0%;P = 0.882)、心血管 (CV) 死亡 (14.3% 对 15.4%;P = 0.429)和全因死亡 (28.7% 对 31.1%;P = 0.217)。5 年时年化缺血性卒中发生率较低,Amulet (1.6%/y) 和 Watchman (1.6%/y) 装置相同。与使用 Watchman 装置的患者相比,使用 Amulet 封堵器的患者中风更轻(n = 38,非致残;n = 11,致残;n = 11,致死;n = 12,未知)。此外,与使用 Amulet 封堵器的患者 (n = 31) 相比,使用 Watchman 装置的患者 (n = 63) 的设备因素 (设备相关血栓或设备周围泄漏 ≥ 3 mm) 更频繁地发生中风事件和 CV 死亡。
结论
最大的随机 LAAO 临床试验的 5 年结果证明了 Amulet 封堵器和 Watchman 2.5 设备的长期安全性和有效性。双密封 Amulet 封堵器可减少与心房颤动相关的血栓栓塞事件,同时无需长期 OAC。(AMPLATZER Amulet 左心耳封堵器 [LAAO] 研究装置豁免 [IDE] 试验 [Amulet IDE 试验];NCT02879448)






























 京公网安备 11010802027423号
京公网安备 11010802027423号