当前位置:
X-MOL 学术
›
Electrochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular insights into the effect of adsorption and reaction of H2O-O2-Cl- on initial electrochemical corrosion of steel
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.electacta.2024.145385 Yumei Nong, Zheng Chen, Ye Chen, Yunchao Tang, Yichen Wang, Kexin Liu, Mingqiang Qin
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.electacta.2024.145385 Yumei Nong, Zheng Chen, Ye Chen, Yunchao Tang, Yichen Wang, Kexin Liu, Mingqiang Qin
|
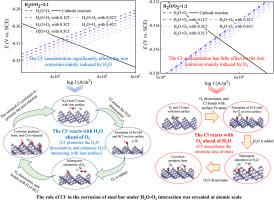
A deeper understanding of the effect of chloride on the process of iron initial corrosion could reveal the detailed mechanism of the Cl--induced acceleration corrosion of steel. In light of the difficulty to unravel the intricacies of H2O-O2-Cl- interactions in experiments, this study conducted the molecular study on the effect of H2O, O2 and Cl- coadsorption and interactions on the electrochemical corrosion process of Fe (100) surface by DFT method, and the electron-correlation functional is GGA-PW91. It is discovered that the adsorption of H2O, O2 and Cl- all promote the electrochemical corrosion of iron. The electrochemical corrosion rate of iron surface rises as the Cl-/H2O concentration ratio increasing, while decrease with the Cl-/O2 concentration ratio increasing. The transition state search found that the presence of Cl- assists the dissociation of H2O on the iron surface, while the interaction between Cl- and O2 could be ignored. Moreover, the successive reaction of Cl-, H2O and O2 with iron surface were further investigated. When the Cl- reacts with H2O ahead of O2, the Cl- promotes the iron oxidation via promoting the dissociation of H2O. While O2 and Cl- firstly coadsorb on iron surface, Cl- accelerates iron oxidation by exacerbating the iron surface losing electrons. These findings could facilitate the development of anti-corrosion techniques for steel in chloride environment.
中文翻译:

H2O-O2-Cl- 吸附和反应对钢初始电化学腐蚀影响的分子见解
更深入地了解氯化物对铁初始腐蚀过程的影响可以揭示 Cl 诱导钢加速腐蚀的详细机制。鉴于实验中难以解开 H2O-O2-Cl- 相互作用的复杂性,本研究采用 DFT 方法对 H2O、O2 和 Cl- 共吸附和相互作用对 Fe (100) 表面电化学腐蚀过程的影响进行了分子研究,电子相关泛函为 GGA-PW91。研究发现,H2O、O2 和 Cl- 的吸附都促进了铁的电化学腐蚀。铁表面的电化学腐蚀速率随 Cl-/H2O 浓度比的增加而增加,而随着 Cl-/O2 浓度比的增加而降低。过渡态搜索发现,Cl- 的存在有助于 H2O 在铁表面的解离,而 Cl- 和 O2 之间的相互作用可以忽略不计。此外,进一步研究了 Cl-、H2O 和 O2 与铁表面的连续反应。当 Cl- 在 O2 之前与 H2O 反应时,Cl- 通过促进 H2O 的解离来促进铁氧化。O2 和 Cl- 首先共吸附在铁表面,而 Cl- 通过加剧铁表面失去电子来加速铁的氧化。这些发现有助于开发氯化物环境中钢的防腐技术。
更新日期:2024-11-19
中文翻译:

H2O-O2-Cl- 吸附和反应对钢初始电化学腐蚀影响的分子见解
更深入地了解氯化物对铁初始腐蚀过程的影响可以揭示 Cl 诱导钢加速腐蚀的详细机制。鉴于实验中难以解开 H2O-O2-Cl- 相互作用的复杂性,本研究采用 DFT 方法对 H2O、O2 和 Cl- 共吸附和相互作用对 Fe (100) 表面电化学腐蚀过程的影响进行了分子研究,电子相关泛函为 GGA-PW91。研究发现,H2O、O2 和 Cl- 的吸附都促进了铁的电化学腐蚀。铁表面的电化学腐蚀速率随 Cl-/H2O 浓度比的增加而增加,而随着 Cl-/O2 浓度比的增加而降低。过渡态搜索发现,Cl- 的存在有助于 H2O 在铁表面的解离,而 Cl- 和 O2 之间的相互作用可以忽略不计。此外,进一步研究了 Cl-、H2O 和 O2 与铁表面的连续反应。当 Cl- 在 O2 之前与 H2O 反应时,Cl- 通过促进 H2O 的解离来促进铁氧化。O2 和 Cl- 首先共吸附在铁表面,而 Cl- 通过加剧铁表面失去电子来加速铁的氧化。这些发现有助于开发氯化物环境中钢的防腐技术。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号