当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fabrication of nitrogen-doped conductive carbon membranes both as anode for degradation of phenol and as cathode for electro-synthesis of hydrogen peroxide in flow-through electrochemical membrane reactor
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.seppur.2024.130586 Yuxuan Zhu, Yinbao Feng, Hong Wang, Jianxin Li
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.seppur.2024.130586 Yuxuan Zhu, Yinbao Feng, Hong Wang, Jianxin Li
|
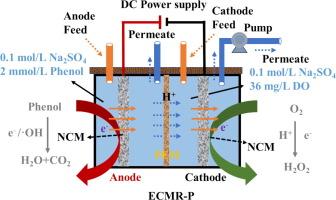
A nitrogen-doped carbon membrane (NCM) was fabricated by high-temperature carbonization utilizing chitosan as precursor, phenolic resin as binder and activated carbon as carbon sources. The impact of the N-AC doping rate (0 − 50 wt%) on the structure and performance of the NCM was investigated. Furthermore, conductive NCM were used to construct an electrochemical membrane reactor with a pair of NCM electrodes (ECMR-P) to simultaneously achieve phenol degradation at the anode and electrosynthesis hydrogen peroxide (H2 O2 ) at the cathode. The results exhibited that NCM50 (50 wt%) had a high porosity (54.2 %) small pore size (22 nm), and high mechanical strength (15.6 MPa) compared to the other NCMs. The ECMR-P with NCM50 as the anode at 2 V achieved a phenol removal rate of up to 99.5 % (the phenol concentration was 2 mmol/L), which is significantly higher than that with NCM0 (43.2 %). Accordingly, total organic carbon (TOC) removal was approximately 13.2 %. Meanwhile, the NCM50 as cathode exhibited a high concentration of H2 O2 electrosynthesis (1236 mg/L) and H2 O2 synthesis rate (6.18 mg/h/cm2 ). The excellent performance of ECMR-P was attributed to the enhanced mass transfer, the abundant pore structure of the NCM, and the electrocatalytic performance enhanced by nitrogen-doped. Density functional theory (DFT) calculations indicated that N-doped improved the adsorption of dissolved oxygen (DO) and phenol, further promoting H2 O2 synthesis and phenol degradation. Furthermore, the collaborative effect of the cathode and anode in ECMR-P enhances hydroxyl radical (·OH) production at the anode and facilitates oxygen reduction reaction (ORR) at the cathode. This is achieved through the diffusion of H+ generated by the anode to the cathode, where it is consumed. Ultimately, this process enhances phenol degradation rate and H2 O2 electrosynthesis. This work demonstrates a promising pathway for achieving water purification and simultaneous in-situ H2 O2 production with high efficiency and safety.
中文翻译:

在流通式电化学膜反应器中制备氮掺杂导电碳膜,既作为苯酚降解的阳极,也作为过氧化氢电合成的阴极
以壳聚糖为前驱体,酚醛树脂为粘结剂,活性炭为碳源,通过高温碳化制备了氮掺杂碳膜 (NCM)。研究了 N-AC 掺杂率 (0 − 50 wt%) 对 NCM 结构和性能的影响。此外,导电 NCM 用于构建具有一对 NCM 电极 (ECMR-P) 的电化学膜反应器,以同时在阳极实现苯酚降解,在阴极实现电合成过氧化氢 (H2O2)。结果表明,与其他 NCM 相比,NCM50 (50 wt%) 具有高孔隙率 (54.2 %)、小孔径 (22 nm) 和高机械强度 (15.6 MPa)。以 NCM50 为阳极,在 2 V 下,ECMR-P 的苯酚去除率高达 99.5 %(苯酚浓度为 2 mmol/L),显著高于 NCM0 的 43.2 %。因此,总有机碳 (TOC) 去除率约为 13.2%。同时,作为阴极的 NCM50 表现出高浓度的 H2O2 电合成 (1236 mg/L) 和 H2O2 合成速率 (6.18 mg/h/cm2)。ECMR-P 的优异性能归因于增强的传质、NCM 丰富的孔隙结构以及氮掺杂增强的电催化性能。密度泛函理论 (DFT) 计算表明,N 掺杂提高了溶解氧 (DO) 和苯酚的吸附,进一步促进了 H2O2 的合成和苯酚降解。此外,ECMR-P 中阴极和阳极的协同作用增强了羟基自由基 (·OH) 的产生,并促进阴极的氧还原反应 (ORR)。这是通过阳极产生的 H+ 扩散到阴极并在那里被消耗来实现的。 最终,该过程提高了苯酚降解速率和 H2O2 电合成。这项工作为实现水净化和高效、安全的同步原位 H2O2 生产提供了一种有前途的途径。
更新日期:2024-11-19
中文翻译:

在流通式电化学膜反应器中制备氮掺杂导电碳膜,既作为苯酚降解的阳极,也作为过氧化氢电合成的阴极
以壳聚糖为前驱体,酚醛树脂为粘结剂,活性炭为碳源,通过高温碳化制备了氮掺杂碳膜 (NCM)。研究了 N-AC 掺杂率 (0 − 50 wt%) 对 NCM 结构和性能的影响。此外,导电 NCM 用于构建具有一对 NCM 电极 (ECMR-P) 的电化学膜反应器,以同时在阳极实现苯酚降解,在阴极实现电合成过氧化氢 (H2O2)。结果表明,与其他 NCM 相比,NCM50 (50 wt%) 具有高孔隙率 (54.2 %)、小孔径 (22 nm) 和高机械强度 (15.6 MPa)。以 NCM50 为阳极,在 2 V 下,ECMR-P 的苯酚去除率高达 99.5 %(苯酚浓度为 2 mmol/L),显著高于 NCM0 的 43.2 %。因此,总有机碳 (TOC) 去除率约为 13.2%。同时,作为阴极的 NCM50 表现出高浓度的 H2O2 电合成 (1236 mg/L) 和 H2O2 合成速率 (6.18 mg/h/cm2)。ECMR-P 的优异性能归因于增强的传质、NCM 丰富的孔隙结构以及氮掺杂增强的电催化性能。密度泛函理论 (DFT) 计算表明,N 掺杂提高了溶解氧 (DO) 和苯酚的吸附,进一步促进了 H2O2 的合成和苯酚降解。此外,ECMR-P 中阴极和阳极的协同作用增强了羟基自由基 (·OH) 的产生,并促进阴极的氧还原反应 (ORR)。这是通过阳极产生的 H+ 扩散到阴极并在那里被消耗来实现的。 最终,该过程提高了苯酚降解速率和 H2O2 电合成。这项工作为实现水净化和高效、安全的同步原位 H2O2 生产提供了一种有前途的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号