当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In(OTf)3-Catalyzed (3 + 3) Dipolar Cyclization of Bicyclo[1.1.0]butanes with N-Nucleophilic 1,3-Dipoles: Access to 2,3-Diazabicyclo[3.1.1]heptanes, 2,3-Diazabicyclo[3.1.1]heptenes, and Enantiopure 2-Azabicyclo[3.1.1]heptanes
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acscatal.4c05622 Jian Zhang, Jia-Yi Su, Hanliang Zheng, Hao Li, Wei-Ping Deng
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acscatal.4c05622 Jian Zhang, Jia-Yi Su, Hanliang Zheng, Hao Li, Wei-Ping Deng
|
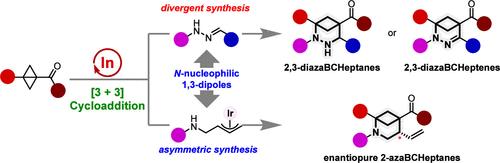
The investigation into the synthesis of azabicyclo[3.1.1]heptanes (azaBCHeps) as bioisosteres to flat aza-aromatics has garnered increasing attention, while it encounters significant challenges. Herein, we have demonstrated the In(OTf)3-catalyzed (3 + 3) dipolar cyclization of bicyclo[1.1.0]butanes (BCBs) with hydrazones and π-allyl-iridium 1,3-dipoles, engendering a diverse array of azaBCHeps. The cyclization of hydrazones and BCBs furnished densely substituted 2,3-diazabicyclo[3.1.1]heptanes and 2,3-diazabicyclo[3.1.1]heptenes under nitrogen and oxygen atmospheres, respectively. A combination of experimental and computational investigations lends robust support for the proton-transfer-interposed sequential mechanism. More importantly, by integrating In(OTf)3/iridium relay catalysis, enantiopure 2-azabicyclo[3.1.1]heptanes were constructed through the (3 + 3) cyclization of BCBs with aza-π-allyl-iridium 1,3-dipoles, in situ generated from N-allyl carbonates. Both methodologies exhibit mild reaction conditions and good tolerance for various functional groups. Moreover, the copious derivatization of products highlights the utility of the newly synthesized heterobicyclic motifs as versatile building blocks in synthetic chemistry.
中文翻译:

在(OTf)3催化的(3 + 3)双环[1.1.0]丁烷与N-亲核1,3-偶极子的偶极环化中:获得2,3-二氮杂双环[3.1.1]庚烷、2,3-二氮杂双环[3.1.1]庚烯和对映体纯2-氮杂双环[3.1.1]庚烷
氮杂双环[3.1.1]庚烷 (azaBCHeps) 作为扁平氮杂芳烃的生物等排体的合成的研究引起了越来越多的关注,同时也遇到了重大挑战。在此,我们证明了 In(OTf)3 催化的 (3 + 3) 双环 [1.1.0] 丁烷 (BCB) 与腙和 π-烯丙基-铱 1,3-偶极子的偶极环化,产生了多种氮杂 BCHep。在氮气和氧气氛下,腙和 BCB 的环化分别提供了密集取代的 2,3-二氮杂双环[3.1.1] 庚烷和 2,3-二氮杂双环[3.1.1] 庚烯。实验和计算研究的结合为质子转移插入序列机制提供了强有力的支持。更重要的是,通过整合In(OTf)3/铱传递催化,通过对映体纯2-氮杂双环[3.1.1]庚烷与氮杂-π-烯丙基-铱1,3-偶极子原位生成对映体纯2-氮杂双环[3.1.1]庚烷。两种方法都表现出温和的反应条件和对各种官能团的良好耐受性。此外,产物的大量衍生化突出了新合成的异双环基序作为合成化学中多功能构建块的实用性。
更新日期:2024-11-19
中文翻译:

在(OTf)3催化的(3 + 3)双环[1.1.0]丁烷与N-亲核1,3-偶极子的偶极环化中:获得2,3-二氮杂双环[3.1.1]庚烷、2,3-二氮杂双环[3.1.1]庚烯和对映体纯2-氮杂双环[3.1.1]庚烷
氮杂双环[3.1.1]庚烷 (azaBCHeps) 作为扁平氮杂芳烃的生物等排体的合成的研究引起了越来越多的关注,同时也遇到了重大挑战。在此,我们证明了 In(OTf)3 催化的 (3 + 3) 双环 [1.1.0] 丁烷 (BCB) 与腙和 π-烯丙基-铱 1,3-偶极子的偶极环化,产生了多种氮杂 BCHep。在氮气和氧气氛下,腙和 BCB 的环化分别提供了密集取代的 2,3-二氮杂双环[3.1.1] 庚烷和 2,3-二氮杂双环[3.1.1] 庚烯。实验和计算研究的结合为质子转移插入序列机制提供了强有力的支持。更重要的是,通过整合In(OTf)3/铱传递催化,通过对映体纯2-氮杂双环[3.1.1]庚烷与氮杂-π-烯丙基-铱1,3-偶极子原位生成对映体纯2-氮杂双环[3.1.1]庚烷。两种方法都表现出温和的反应条件和对各种官能团的良好耐受性。此外,产物的大量衍生化突出了新合成的异双环基序作为合成化学中多功能构建块的实用性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号