当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of Advanced Gene Delivery Carriers: Macrophage Phenotype Matters
Advanced Materials ( IF 27.4 ) Pub Date : 2024-11-19 , DOI: 10.1002/adma.202401504 Yue Wang, Yining Yao, Yue Zhang, Yingjie Yu, Jiangqi Luo, Matthew J. Sweet, Chengzhong Yu
Advanced Materials ( IF 27.4 ) Pub Date : 2024-11-19 , DOI: 10.1002/adma.202401504 Yue Wang, Yining Yao, Yue Zhang, Yingjie Yu, Jiangqi Luo, Matthew J. Sweet, Chengzhong Yu
|
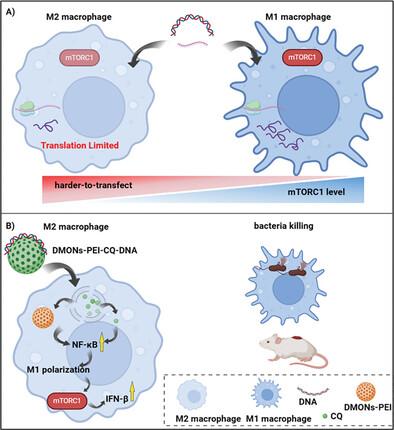
Nucleic acid delivery in hard‐to‐transfect macrophages have attracted increasing attention in diverse applications such as defence against bacterial infection. Regulated by microenvironments in specific applications, macrophages have a heterogenous nature and exist in different phenotypes with diverse functions, e.g., pro‐inflammatory and anti‐inflammatory. However, it is not clear whether macrophage phenotype affects nucleic acid delivery, and which one is harder to transfect, and the design of nucleic acid carriers in harder‐to‐transfect macrophage phenotypes is largely unexplored. Herein, it is first revealed that nucleic acid delivery efficacy in macrophages is influenced by phenotype: IL‐4‐treated “M2‐like” macrophages with suppressed mammalian target of rapamycin complex 1 (mTORC1) levels are harder‐to‐transfect than “M1‐like” macrophages for mRNA and DNA. This knowledge is then translated to the purpose‐design of gene delivery carriers for harder‐to‐transfect M2 phenotype macrophages dominant upon bacteria immune evasion. By loading chloroquine in tetrasulfide bond‐containing organosilica nanoparticles, the resultant composite promotes macrophage M2 polarization to M1 and increases mTORC1 levels for enhanced translation. The design is demonstrated in vitro and in vivo for pathogenic Escherichia coli (E. coli ) and methicillin‐resistant Staphylococcus aureus (MRSA) infections. It is expected that the findings may provide new knowledge and gene delivery solutions in other applications where the M2 phenotype macrophage is dominant.
中文翻译:

高级基因递送载体的合理设计:巨噬细胞表型很重要
难转染巨噬细胞中的核酸递送在防御细菌感染等各种应用中引起了越来越多的关注。巨噬细胞在特定应用中受微环境的调节,具有异质性,以不同的表型存在,具有不同的功能,例如促炎和抗炎。然而,目前尚不清楚巨噬细胞表型是否会影响核酸递送,以及哪种更难转染,并且更难转染的巨噬细胞表型中的核酸载体设计在很大程度上尚未探索。在此,首次揭示了巨噬细胞中的核酸递送功效受表型的影响:IL-4 处理的“M2 样”巨噬细胞具有抑制的哺乳动物雷帕霉素复合物 1 (mTORC1) 水平比“M1 样”巨噬细胞更难转染 mRNA 和 DNA。然后将这些知识转化为基因递送载体的用途设计,用于在细菌免疫逃避方面占主导地位的更难转染的 M2 表型巨噬细胞。通过将氯喹加载到含有四硫键的有机硅纳米颗粒中,所得复合物促进巨噬细胞 M2 极化为 M1 并增加 mTORC1 水平以增强翻译。该设计在体外和体内证明了致病性大肠埃希菌 (E. coli) 和耐甲氧西林金黄色葡萄球菌 (MRSA) 感染。预计这些发现可能会在 M2 表型巨噬细胞占主导地位的其他应用中提供新的知识和基因递送解决方案。
更新日期:2024-11-19
中文翻译:

高级基因递送载体的合理设计:巨噬细胞表型很重要
难转染巨噬细胞中的核酸递送在防御细菌感染等各种应用中引起了越来越多的关注。巨噬细胞在特定应用中受微环境的调节,具有异质性,以不同的表型存在,具有不同的功能,例如促炎和抗炎。然而,目前尚不清楚巨噬细胞表型是否会影响核酸递送,以及哪种更难转染,并且更难转染的巨噬细胞表型中的核酸载体设计在很大程度上尚未探索。在此,首次揭示了巨噬细胞中的核酸递送功效受表型的影响:IL-4 处理的“M2 样”巨噬细胞具有抑制的哺乳动物雷帕霉素复合物 1 (mTORC1) 水平比“M1 样”巨噬细胞更难转染 mRNA 和 DNA。然后将这些知识转化为基因递送载体的用途设计,用于在细菌免疫逃避方面占主导地位的更难转染的 M2 表型巨噬细胞。通过将氯喹加载到含有四硫键的有机硅纳米颗粒中,所得复合物促进巨噬细胞 M2 极化为 M1 并增加 mTORC1 水平以增强翻译。该设计在体外和体内证明了致病性大肠埃希菌 (E. coli) 和耐甲氧西林金黄色葡萄球菌 (MRSA) 感染。预计这些发现可能会在 M2 表型巨噬细胞占主导地位的其他应用中提供新的知识和基因递送解决方案。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号