当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coordination Induced Spin State Transition Switches the Reactivity of Nickel (II) Porphyrin in Hydrogen Evolution Reaction
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-11-19 , DOI: 10.1002/anie.202413042
Hao-Zong Xue 1 , Jia-Hui Wu 1 , Bing-Wu Wang 1 , Song Gao 1, 2 , Jun-Long Zhang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-11-19 , DOI: 10.1002/anie.202413042
Hao-Zong Xue 1 , Jia-Hui Wu 1 , Bing-Wu Wang 1 , Song Gao 1, 2 , Jun-Long Zhang 1
Affiliation
|
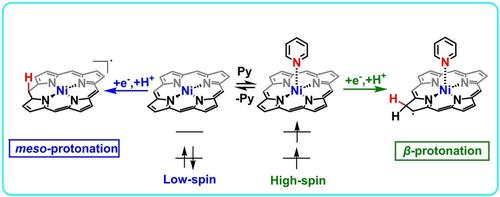
The study explores the impact of coordination-induced spin-state switching on hydrogen evolution reaction activity of nickel(II) porphyrin, revealing that low-spin Ni(II) porphyrin favors hydrogen evolution while high-spin counterpart facilitates β-hydrogenation of porphyrin periphery. Our finding highlights the pivotal role of spin transition in regulating the reactivity and selectivity of metal-catalyzed reactions.
中文翻译:

配位诱导的自旋态转变开关镍 (II) 卟啉在析氢反应中的反应性
该研究探讨了配位诱导的自旋态切换对镍 (II) 卟啉析氢反应活性的影响,揭示了低自旋 Ni(II) 卟啉有利于析氢,而高自旋对应物促进卟啉外围的β氢化。我们的研究结果强调了自旋跃迁在调节金属催化反应的反应性和选择性中的关键作用。
更新日期:2024-11-19
中文翻译:

配位诱导的自旋态转变开关镍 (II) 卟啉在析氢反应中的反应性
该研究探讨了配位诱导的自旋态切换对镍 (II) 卟啉析氢反应活性的影响,揭示了低自旋 Ni(II) 卟啉有利于析氢,而高自旋对应物促进卟啉外围的β氢化。我们的研究结果强调了自旋跃迁在调节金属催化反应的反应性和选择性中的关键作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号