Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
EGCG‐enabled Deep Tumor Penetration of Phosphatase and Acidity Dual‐responsive Nanotherapeutics for Combinatory Therapy of Breast Cancer
Small ( IF 13.0 ) Pub Date : 2024-11-19 , DOI: 10.1002/smll.202406245 Mengxue Zhou, Chuang Zhou, Huan Geng, Zhiwei Huang, Zhiyuan Lin, Ying Wang, Yin Zhu, Jiang Shi, Junfeng Tan, Li Guo, Yanni Zhao, Yue Zhang, Qunhua Peng, Haijun Yu, Weidong Dai, Haipeng Lv, Zhi Lin
Small ( IF 13.0 ) Pub Date : 2024-11-19 , DOI: 10.1002/smll.202406245 Mengxue Zhou, Chuang Zhou, Huan Geng, Zhiwei Huang, Zhiyuan Lin, Ying Wang, Yin Zhu, Jiang Shi, Junfeng Tan, Li Guo, Yanni Zhao, Yue Zhang, Qunhua Peng, Haijun Yu, Weidong Dai, Haipeng Lv, Zhi Lin
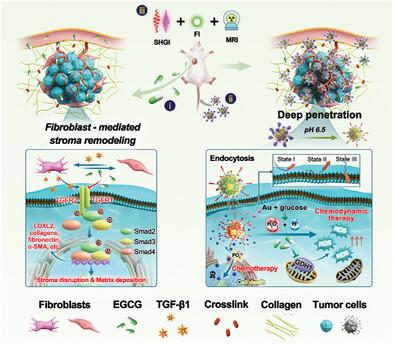
|
The presence of dense collagen fibers is a typical characteristic of triple‐negative breast cancer (TNBC). Although these fibers hinder drug penetration and reduce treatment efficacy, the depletion of the collagen matrix is associated with tumor metastasis. To address this issue, epigallocatechin‐3‐gallate (EGCG) is first exploited for disrupting the dense collagenous stroma and alleviate fibrosis by specifically blocking the TGF‐β/Smad pathway in fibroblasts and tumor cells when intraperitoneally administrated in TNBC tumor‐bearing mice. A methotrexate (MTX)‐loaded dual phosphate‐ and pH‐responsive nanodrug (pHA@MOF‐Au/MTX) is next engineered by integrating Fe‐based metal–organic frameworks and gold nanoparticles for improved chemo/chemodynamic therapy of TNBC. Surface modification with pH (low)‐insertion peptide substantially enhanced the binding of the nanodrug to 4T1 cells owing to tumor stroma remodeling by EGCG. High‐concentration EGCG inhibited glutathione peroxidase by regulating mitochondrial glutamine metabolism, thus facilitating tumor cell ferroptosis. Furthermore, sequential EGCG and pHA@MOF‐Au/MTX treatment showed remarkable anti‐tumor effects in a mouse model of TNBC, with a tumor growth inhibition rate of 79.9%, and a pulmonary metastasis rate of 96.8%. Altogether, the combination strategy developed in this study can improve the efficacy of chemo/chemodynamic therapy in TNBC and represents an innovative application of EGCG.
中文翻译:

EGCG 使磷酸酶和酸度的深部肿瘤渗透:用于乳腺癌联合治疗的双反应纳米疗法
致密胶原纤维的存在是三阴性乳腺癌 (TNBC) 的典型特征。尽管这些纤维阻碍了药物渗透并降低了治疗效果,但胶原蛋白基质的耗竭与肿瘤转移有关。为了解决这个问题,表没食子儿茶素-3-没食子酸酯 (EGCG) 首先被用于破坏致密的胶原基质,并通过特异性阻断成纤维细胞和肿瘤细胞中的 TGF - β/Smad 通路来缓解纤维化在TNBC荷瘤小鼠中腹膜内给药。接下来,通过整合铁基金属有机框架和金纳米颗粒来设计载有甲氨蝶呤 (MTX) 的双重磷酸盐和 pH 反应纳米药物 (pHA@MOF-Au/MTX),以改善 TNBC 的化学/化学动力学治疗。由于 EGCG 重塑肿瘤基质,用 pH (低) 插入肽进行表面修饰显着增强了纳米药物与 4T1 细胞的结合。高浓度 EGCG 通过调节线粒体谷氨酰胺代谢抑制谷胱甘肽过氧化物酶,从而促进肿瘤细胞铁死亡。此外,在 TNBC 小鼠模型中,序贯 EGCG 和 pHA@MOF-Au/MTX 治疗显示出显著的抗肿瘤作用,肿瘤生长抑制率为 79.9%,肺转移率为 96.8%。总而言之,本研究开发的联合策略可以提高 TNBC 化疗/化疗动力学疗法的疗效,代表了 EGCG 的创新应用。
更新日期:2024-11-19
中文翻译:

EGCG 使磷酸酶和酸度的深部肿瘤渗透:用于乳腺癌联合治疗的双反应纳米疗法
致密胶原纤维的存在是三阴性乳腺癌 (TNBC) 的典型特征。尽管这些纤维阻碍了药物渗透并降低了治疗效果,但胶原蛋白基质的耗竭与肿瘤转移有关。为了解决这个问题,表没食子儿茶素-3-没食子酸酯 (EGCG) 首先被用于破坏致密的胶原基质,并通过特异性阻断成纤维细胞和肿瘤细胞中的 TGF - β/Smad 通路来缓解纤维化在TNBC荷瘤小鼠中腹膜内给药。接下来,通过整合铁基金属有机框架和金纳米颗粒来设计载有甲氨蝶呤 (MTX) 的双重磷酸盐和 pH 反应纳米药物 (pHA@MOF-Au/MTX),以改善 TNBC 的化学/化学动力学治疗。由于 EGCG 重塑肿瘤基质,用 pH (低) 插入肽进行表面修饰显着增强了纳米药物与 4T1 细胞的结合。高浓度 EGCG 通过调节线粒体谷氨酰胺代谢抑制谷胱甘肽过氧化物酶,从而促进肿瘤细胞铁死亡。此外,在 TNBC 小鼠模型中,序贯 EGCG 和 pHA@MOF-Au/MTX 治疗显示出显著的抗肿瘤作用,肿瘤生长抑制率为 79.9%,肺转移率为 96.8%。总而言之,本研究开发的联合策略可以提高 TNBC 化疗/化疗动力学疗法的疗效,代表了 EGCG 的创新应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号