当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Adsorption of Alkaline Cations Enhances CO–CO Coupling in CO2 Electroreduction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-18 , DOI: 10.1021/jacs.4c10455 Yanyang Qin, Chenfeng Xia, Tiantian Wu, Jianrui Zhang, Guoxin Gao, Bao Yu Xia, Michelle L. Coote, Shujiang Ding, Yaqiong Su
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-18 , DOI: 10.1021/jacs.4c10455 Yanyang Qin, Chenfeng Xia, Tiantian Wu, Jianrui Zhang, Guoxin Gao, Bao Yu Xia, Michelle L. Coote, Shujiang Ding, Yaqiong Su
|
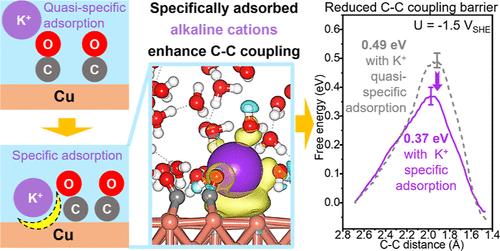
Electrolyte alkaline cations can significantly modulate the reaction selectivity of electrochemical CO2 reduction (eCO2R), enhancing the yield of the valuable multicarbon (C2+) chemical feedstocks. However, the mechanism underlying this cation effect on the C–C coupling remains unclear. Herein, by performing constant-potential AIMD simulations, we studied the dynamic behavior of interfacial K+ ions over Cu surfaces during C–C coupling and the origin of the cation effect. We showed that the specific adsorption of K+ readily occurs at the surface sites adjacent to the *CO intermediates on the Cu surfaces. Furthermore, this specific adsorption of K+ during *CO–*CO coupling is more important than quasi-specific adsorption for enhancing coupling kinetics, reducing the coupling barriers by approximately 0.20 eV. Electronic structure analysis revealed that charge redistribution occurs between the specifically adsorbed K+, *CO, and Cu sites, and this can account for the reduced barriers. In addition, we identified excellent *CO–*CO coupling selectivity on Cu(100) with K+ ions. Experimental results show that suppressing surface K+-specific adsorption using the surfactant cetyltrimethylammonium bromide (CTAB) significantly decreases the Faradaic efficiency for C2 products from 41.1% to 4.3%, consistent with our computational findings. This study provides crucial insights for improving the selectivity toward C2+ products by rationally tuning interfacial cation adsorption during eCO2R. Specifically, C–C coupling can be enhanced by promoting K+-specific adsorption, for example, by confining K+ within a coated layer or using pulsed negative potentials.
中文翻译:

碱性阳离子的特异性吸附增强了 CO2 电还原中的 CO-CO 偶联
电解质碱性阳离子可以显着调节电化学 CO2 还原 (eCO2R) 的反应选择性,从而提高有价值的多碳 (C2+) 化学原料的产量。然而,这种阳离子效应对 C-C 偶联的潜在机制仍不清楚。在此,通过进行恒定电位 AIMD 模拟,我们研究了 C-C 耦合过程中界面 K+ 离子在 Cu 表面上的动态行为以及阳离子效应的起源。我们表明,K+ 的特异性吸附很容易发生在 Cu 表面上与 *CO 中间体相邻的表面位点。此外,在 *CO–*CO 耦合过程中 K+ 的这种特异性吸附对于增强耦合动力学来说比准特异性吸附更重要,将耦合势垒减少约 0.20 eV。电子结构分析表明,电荷重新分布在特异性吸附的 K+、*CO 和 Cu 位点之间,这可以解释势垒减少的原因。此外,我们还确定了对 Cu(100) 与 K+ 离子的出色 *CO–*CO 偶联选择性。实验结果表明,使用表面活性剂十六烷基三甲基溴化铵 (CTAB) 抑制表面 K+ 特异性吸附可将 C2 产物的法拉第效率从 41.1% 降低到 4.3%,这与我们的计算结果一致。本研究通过合理调节 eCO2R 过程中的界面阳离子吸附,为提高对 C2+ 产物的选择性提供了重要见解。 具体来说,可以通过促进 K+ 特异性吸附来增强 C-C 耦合,例如,通过将 K+ 限制在涂层内或使用脉冲负电位。
更新日期:2024-11-18
中文翻译:

碱性阳离子的特异性吸附增强了 CO2 电还原中的 CO-CO 偶联
电解质碱性阳离子可以显着调节电化学 CO2 还原 (eCO2R) 的反应选择性,从而提高有价值的多碳 (C2+) 化学原料的产量。然而,这种阳离子效应对 C-C 偶联的潜在机制仍不清楚。在此,通过进行恒定电位 AIMD 模拟,我们研究了 C-C 耦合过程中界面 K+ 离子在 Cu 表面上的动态行为以及阳离子效应的起源。我们表明,K+ 的特异性吸附很容易发生在 Cu 表面上与 *CO 中间体相邻的表面位点。此外,在 *CO–*CO 耦合过程中 K+ 的这种特异性吸附对于增强耦合动力学来说比准特异性吸附更重要,将耦合势垒减少约 0.20 eV。电子结构分析表明,电荷重新分布在特异性吸附的 K+、*CO 和 Cu 位点之间,这可以解释势垒减少的原因。此外,我们还确定了对 Cu(100) 与 K+ 离子的出色 *CO–*CO 偶联选择性。实验结果表明,使用表面活性剂十六烷基三甲基溴化铵 (CTAB) 抑制表面 K+ 特异性吸附可将 C2 产物的法拉第效率从 41.1% 降低到 4.3%,这与我们的计算结果一致。本研究通过合理调节 eCO2R 过程中的界面阳离子吸附,为提高对 C2+ 产物的选择性提供了重要见解。 具体来说,可以通过促进 K+ 特异性吸附来增强 C-C 耦合,例如,通过将 K+ 限制在涂层内或使用脉冲负电位。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号