当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Cycloaddition of Olefins with In Situ Generated N-Boc-Formaldimine
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-18 , DOI: 10.1021/jacs.4c13538 Marian Guillén, Markus Leutzsch, Benjamin List
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-18 , DOI: 10.1021/jacs.4c13538 Marian Guillén, Markus Leutzsch, Benjamin List
|
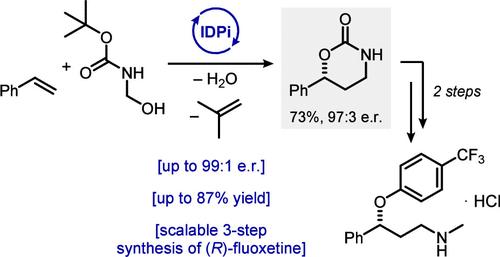
Chiral 1,3-amino alcohols are ubiquitous structural motifs in natural products and active pharmaceutical ingredients. We present a highly enantioselective, inverse-electron-demand hetero-Diels–Alder reaction of olefins with in situ generated N-Boc-formaldimine catalyzed by strong and confined Bro̷nsted acids. This transformation provides direct access to valuable 1,3-amino alcohols from styrenes and 1,1-disubtituted alkenes. Isotope labeling studies and kinetic analysis reveal an unusual mechanism involving an oxazinium intermediate and a catalyst order greater than one.
中文翻译:

烯烃与原位生成的 N-Boc-Formaldimine 的催化不对称环加成反应
手性 1,3-氨基醇是天然产物和活性药物成分中普遍存在的结构基序。我们提出了烯烃与原位生成的 N-Boc-formaldimine 在强和限制 Bro̷nsted 酸催化下发生的高对映选择性、逆电子需求异质 Diels-Alder 反应。这种转化提供了从苯乙烯和 1,1-二取代烯烃中直接获得有价值的 1,3-氨基醇的机会。同位素标记研究和动力学分析揭示了一种涉及恶嗪中间体和大于 1 的催化剂级数的不寻常机制。
更新日期:2024-11-19
中文翻译:

烯烃与原位生成的 N-Boc-Formaldimine 的催化不对称环加成反应
手性 1,3-氨基醇是天然产物和活性药物成分中普遍存在的结构基序。我们提出了烯烃与原位生成的 N-Boc-formaldimine 在强和限制 Bro̷nsted 酸催化下发生的高对映选择性、逆电子需求异质 Diels-Alder 反应。这种转化提供了从苯乙烯和 1,1-二取代烯烃中直接获得有价值的 1,3-氨基醇的机会。同位素标记研究和动力学分析揭示了一种涉及恶嗪中间体和大于 1 的催化剂级数的不寻常机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号