当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Octadentate Bispidine Chelators for Tb(III) Complexation: Pyridine Carboxylate versus Pyridine Phosphonate Donors
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.inorgchem.4c03691 Lucas Petitpoisson, Anli Mahamoud, Valérie Mazan, Maryame Sy, Olivier Jeannin, Eva Tóth, Loïc J. Charbonnière, Mourad Elhabiri, Aline M. Nonat
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.inorgchem.4c03691 Lucas Petitpoisson, Anli Mahamoud, Valérie Mazan, Maryame Sy, Olivier Jeannin, Eva Tóth, Loïc J. Charbonnière, Mourad Elhabiri, Aline M. Nonat
|
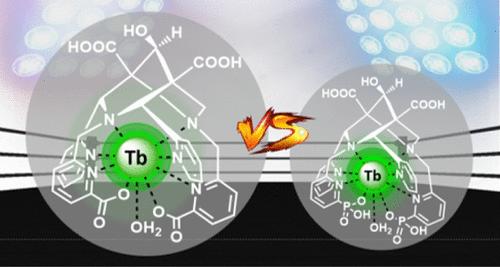
With their rigid and preorganized skeleton, bispidine (3,7-diazabicyclo[3.3.1]nonane) chelators are very appealing for the preparation of metal complexes with high kinetic inertness. With the aim to develop new Tb(III)-based medical imaging probes, this study describes the synthesis and physicochemical properties of two novel terbium(III) complexes with octadentate bispidine-based ligands substituted with either pyridine-phosphonate (H6L1) or picolinate (H4L2) subunits. Thermodynamic stability constants of the corresponding Tb(III) complexes have been determined by potentiometric, UV–visible absorption spectrophotometric and spectrofluorimetric methods. Despite their apparent similarity, these two octadentate ligands differ in their most stable conformation: chair–chair conformation for H4L2 and boat–chair conformation for H6L1, as confirmed by 1H NMR studies and suggested by physicochemical investigations. This conformational change induces different protonation schemes for the two ligands. The kinetic inertness of the Tb complexes has been studied in various media and assessed by transmetalation and transchelation experiments. In particular, Tb(L2) displayed a remarkable kinetic inertness with no measurable dissociation over two months in mouse serum at 10–5 M concentration. The complex was also very inert in the presence of a 50-fold excess of Zn(II) in H2O at pH = 7.4 (7% of dissociation over two months). The complexes with ligand L1 are significantly less inert, emphasizing the influence of the ligand conformation on the kinetic inertness of the Ln(III) complexes. Finally, the luminescence properties of the isolated complexes have also been investigated. A bright green luminescence was observed, especially for Tb(L2), which displays a high quantum yield value of 50% in H2O (60% in D2O; λexc = 263 nm). In addition, luminescence lifetimes of 1.9(2) and 1.7(2) ms have been measured for Tb(L1) and Tb(L2), respectively, hence confirming the formation of nona-coordinated complexes with one inner-sphere water molecule. These data on a bispidine scaffold pave the way for developing bright, inert luminescent probes for bioimaging and for radiolabeling applications with Tb(III) radioisotopes.
中文翻译:

用于 Tb(III) 络合的八齿二螺汀螯合剂:羧酸吡啶与膦酸盐吡啶供体的比较
Bispidine(3,7-二氮杂双环[3.3.1]壬烷)螯合剂具有刚性和预组织化的骨架,对于制备具有高动力学惰性的金属络合物非常有吸引力。为了开发基于 Tb(III) 的新型医学成像探针,本研究描述了两种新型铽 (III) 配合物的合成和物理化学性质,其中八齿二螺定配体被吡啶-膦酸盐 (H6L1) 或吡啶酸盐 (H4L2 ) 取代) 子单位。相应的 Tb(III) 配合物的热力学稳定性常数已通过电位法、紫外-可见吸收分光光度法和分光荧光法测定。尽管它们表面上相似,但这两种八齿配体在最稳定的构象上有所不同:H4L2 的椅子-椅子构象和 H6L1 的船-椅子构象,1 HNMR 研究证实并由物理化学调查表明。这种构象变化诱导了两种配体的不同质子化方案。已在各种培养基中研究了 Tb 复合物的动力学惰性,并通过转金属化和转螯合实验进行了评估。特别是,Tb(L2) 在 10-5 M 浓度的小鼠血清中表现出显著的动力学惰性,在两个月内没有可测量的解离。在 pH = 7.4 时,当 H2O 中 Zn(II) 过量 50 倍时,该复合物也具有很强的惰性(两个月内解离的 7%)。 与配体 L1 的复合物的惰性明显较低,强调了配体构象对 Ln(III) 复合物动力学惰性的影响。最后,还研究了分离的复合物的发光特性。观察到亮绿色发光,尤其是 Tb(L2),它在 H2O 中显示出 50% 的高量子产率值(在 D2O 中为 60%;λexc = 263 nm)。此外,已经分别测量了 Tb(L1) 和 Tb(L2) 的 1.9(2) 和 1.7(2) ms 的发光寿命,从而证实了与一个内球水分子形成九位络合物。Bispidine 支架上的这些数据为开发用于生物成像和 Tb(III) 放射性同位素放射性标记应用的明亮、惰性发光探针铺平了道路。
更新日期:2024-11-19
中文翻译:

用于 Tb(III) 络合的八齿二螺汀螯合剂:羧酸吡啶与膦酸盐吡啶供体的比较
Bispidine(3,7-二氮杂双环[3.3.1]壬烷)螯合剂具有刚性和预组织化的骨架,对于制备具有高动力学惰性的金属络合物非常有吸引力。为了开发基于 Tb(III) 的新型医学成像探针,本研究描述了两种新型铽 (III) 配合物的合成和物理化学性质,其中八齿二螺定配体被吡啶-膦酸盐 (H6L1) 或吡啶酸盐 (H4L2 ) 取代) 子单位。相应的 Tb(III) 配合物的热力学稳定性常数已通过电位法、紫外-可见吸收分光光度法和分光荧光法测定。尽管它们表面上相似,但这两种八齿配体在最稳定的构象上有所不同:H4L2 的椅子-椅子构象和 H6L1 的船-椅子构象,1 HNMR 研究证实并由物理化学调查表明。这种构象变化诱导了两种配体的不同质子化方案。已在各种培养基中研究了 Tb 复合物的动力学惰性,并通过转金属化和转螯合实验进行了评估。特别是,Tb(L2) 在 10-5 M 浓度的小鼠血清中表现出显著的动力学惰性,在两个月内没有可测量的解离。在 pH = 7.4 时,当 H2O 中 Zn(II) 过量 50 倍时,该复合物也具有很强的惰性(两个月内解离的 7%)。 与配体 L1 的复合物的惰性明显较低,强调了配体构象对 Ln(III) 复合物动力学惰性的影响。最后,还研究了分离的复合物的发光特性。观察到亮绿色发光,尤其是 Tb(L2),它在 H2O 中显示出 50% 的高量子产率值(在 D2O 中为 60%;λexc = 263 nm)。此外,已经分别测量了 Tb(L1) 和 Tb(L2) 的 1.9(2) 和 1.7(2) ms 的发光寿命,从而证实了与一个内球水分子形成九位络合物。Bispidine 支架上的这些数据为开发用于生物成像和 Tb(III) 放射性同位素放射性标记应用的明亮、惰性发光探针铺平了道路。































 京公网安备 11010802027423号
京公网安备 11010802027423号