当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Tandem-Locked Fluorescent Probe Activated by Hypoxia and a Viscous Environment for Precise Intraoperative Imaging of Tumor and Instant Assessment of Ferroptosis-Mediated Therapy
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.analchem.4c04820 Jiao Lu, Guiling Zhao, Yonghai Wang, Rui Wang, Yanlong Xing, Fabiao Yu, Kun Dou
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.analchem.4c04820 Jiao Lu, Guiling Zhao, Yonghai Wang, Rui Wang, Yanlong Xing, Fabiao Yu, Kun Dou
|
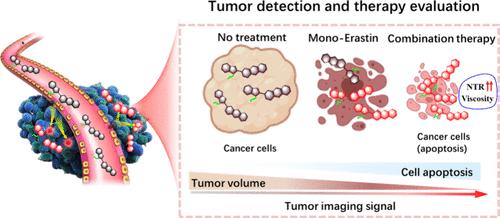
Noninvasive fluorescence detection of tumor-associated biomarker dynamics provides immediate insights into tumor biology, which are essential for assessing the efficacy of therapeutic interventions, adapting treatment strategies, and achieving personalized diagnosis and therapy evaluation. However, due to the absence of a single biomarker that effectively reflects tumor development and progression, the currently available optical diagnostic agents that rely on “always-on” or single pathological activation frequently show nonspecific fluorescence responses and limited tumor accumulation, which inevitably compromises the accuracy and reliability of tumor imaging. Herein, based on intramolecular charge transfer (ICT) and twisted intramolecular charge-transfer (TICT) hybrid mechanisms, we report a tandem-locked probe, NTVI-Biotin, for simultaneously specific imaging-guided tumor resection and ferroptosis-mediated tumor ablation evaluation under the coactivation of nitro reductase (NTR)/viscosity. The dual-stimulus-responsive design strategy ensures that NTVI-Biotin exclusively activates near-infrared (NIR) fluorescence signals upon interaction with both NTR and elevated viscosity levels through triggering ICT on while inhibiting the TICT process. Meanwhile, functionalization with a tumor-targeting hydrophilic biotin-poly(ethylene glycol) moiety enhances tumor accumulation. The probe’s dual-response and tumor-targeting design minimizes nonspecific tissue activation, allowing for precise tumor identification and lesion removal with a superior tumor-to-normal tissue (T/N > 6) ratio. More importantly, NTVI-Biotin was capable of evaluating ferroptosis-mediated chemotherapeutics by real-time monitoring of the alternations of NTR/viscosity levels. The results reveal that the increased tumor signals of NTVI-Biotin following the combination of ferroptosis and chemotherapy correlate well with the tumor growth inhibition, demonstrating the potential of NTVI-Biotin to assess therapeutic efficacy.
中文翻译:

由缺氧和粘性环境激活的串联锁定荧光探针,用于肿瘤的精确术中成像和铁死亡介导的治疗的即时评估
肿瘤相关生物标志物动力学的无创荧光检测提供了对肿瘤生物学的即时见解,这对于评估治疗干预的疗效、调整治疗策略以及实现个性化诊断和治疗评估至关重要。然而,由于缺乏有效反映肿瘤发展和进展的单一生物标志物,目前可用的依赖于“始终开启”或单一病理激活的光学诊断试剂经常表现出非特异性荧光反应和有限的肿瘤积累,这不可避免地影响了肿瘤成像的准确性和可靠性。在此,基于分子内电荷转移 (ICT) 和扭曲的分子内电荷转移 (TICT) 杂交机制,我们报道了一种串联锁定探针 NTVI-生物素,用于在硝基还原酶 (NTR) /粘度的共激活下同时特异性成像引导的肿瘤切除和铁死亡介导的肿瘤消融评估。双重刺激响应设计策略确保 NTVI-生物素在与 NTR 和升高的粘度水平相互作用时通过触发 ICT 同时抑制 TICT 过程,专门激活近红外 (NIR) 荧光信号。同时,使用靶向肿瘤的亲水性生物素-聚(乙二醇)部分的功能化可增强肿瘤积累。该探针的双反应和肿瘤靶向设计最大限度地减少了非特异性组织活化,从而以优异的肿瘤与正常组织 (T/N > 6) 比率实现精确的肿瘤识别和病灶去除。更重要的是,NTVI-Biotin 能够通过实时监测 NTR/粘度水平的变化来评估铁死亡介导的化疗药物。 结果表明,铁死亡和化疗联合后 NTVI-Biotin 肿瘤信号增加与肿瘤生长抑制密切相关,证明了 NTVI-Biotin 评估治疗效果的潜力。
更新日期:2024-11-19
中文翻译:

由缺氧和粘性环境激活的串联锁定荧光探针,用于肿瘤的精确术中成像和铁死亡介导的治疗的即时评估
肿瘤相关生物标志物动力学的无创荧光检测提供了对肿瘤生物学的即时见解,这对于评估治疗干预的疗效、调整治疗策略以及实现个性化诊断和治疗评估至关重要。然而,由于缺乏有效反映肿瘤发展和进展的单一生物标志物,目前可用的依赖于“始终开启”或单一病理激活的光学诊断试剂经常表现出非特异性荧光反应和有限的肿瘤积累,这不可避免地影响了肿瘤成像的准确性和可靠性。在此,基于分子内电荷转移 (ICT) 和扭曲的分子内电荷转移 (TICT) 杂交机制,我们报道了一种串联锁定探针 NTVI-生物素,用于在硝基还原酶 (NTR) /粘度的共激活下同时特异性成像引导的肿瘤切除和铁死亡介导的肿瘤消融评估。双重刺激响应设计策略确保 NTVI-生物素在与 NTR 和升高的粘度水平相互作用时通过触发 ICT 同时抑制 TICT 过程,专门激活近红外 (NIR) 荧光信号。同时,使用靶向肿瘤的亲水性生物素-聚(乙二醇)部分的功能化可增强肿瘤积累。该探针的双反应和肿瘤靶向设计最大限度地减少了非特异性组织活化,从而以优异的肿瘤与正常组织 (T/N > 6) 比率实现精确的肿瘤识别和病灶去除。更重要的是,NTVI-Biotin 能够通过实时监测 NTR/粘度水平的变化来评估铁死亡介导的化疗药物。 结果表明,铁死亡和化疗联合后 NTVI-Biotin 肿瘤信号增加与肿瘤生长抑制密切相关,证明了 NTVI-Biotin 评估治疗效果的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号