当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Cell Surface Glycoproteins Using Enzymatic Treatment and Mass Spectrometry
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.analchem.4c04286 Ding Chiao Lin, T. Mamie Lih, Hongyi Liu, Hui Zhang
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.analchem.4c04286 Ding Chiao Lin, T. Mamie Lih, Hongyi Liu, Hui Zhang
|
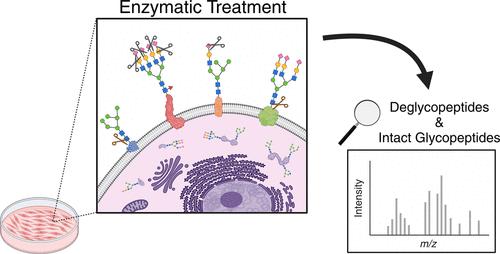
Almost all proteins on the cell surface are modified by glycosylation. Cell surface glycoproteins participate in various cellular pathways, such as cell adhesion, cell–cell communication, and immune response. Due to their functional importance, glycoproteins on the cell surface often serve as potential therapeutic targets. Recent advancements in mass spectrometry (MS) have facilitated the characterization of glycoproteins that are generally localized on the cell surface, secreted to the extracellular environment, or found in intracellular organelles such as the endoplasmic reticulum, Golgi apparatus, and peroxisome. However, the selective characterization of glycoproteins on the cell surface remains challenging. In this study, we applied enzymatic treatment to live cells, followed by MS-based glycoproteomics analysis, to assess changes in protein glycosylation at different treatment time points as a method to identify cell surface glycoproteins. To demonstrate this approach, a renal cell carcinoma cell line, A498, was treated with glycosidases, sialidase and PNGase F, over two treatment time intervals, 2 and 24 h. Glycoproteins were identified as cell surface glycoproteins from A498 cells when enzyme treatment altered the glycosylation of the glycoproteins. The results revealed the effectiveness of integrating enzymatic treatment with MS-based glycoproteomics for analyzing cell surface glycoproteins. Our established method has demonstrated the potential applications for assessing accessibility of therapeutic targets on the cell surface over time and supporting the development of new targeted therapies.
中文翻译:

使用酶处理和质谱法表征细胞表面糖蛋白
细胞表面几乎所有的蛋白质都通过糖基化修饰。细胞表面糖蛋白参与各种细胞途径,例如细胞粘附、细胞间通讯和免疫反应。由于其功能重要性,细胞表面的糖蛋白通常作为潜在的治疗靶标。质谱 (MS) 的最新进展有助于表征通常位于细胞表面、分泌到细胞外环境中或在细胞内细胞器(如内质网、高尔基体和过氧化物酶体)中发现的糖蛋白。然而,细胞表面糖蛋白的选择性表征仍然具有挑战性。在这项研究中,我们对活细胞进行酶处理,然后进行基于 MS 的糖蛋白质组学分析,以评估不同处理时间点蛋白质糖基化的变化,作为鉴定细胞表面糖蛋白的方法。为了证明这种方法,在两个治疗时间间隔(2 小时和 24 小时)内用糖苷酶、唾液酸酶和 PNGase F 处理肾细胞癌细胞系 A498。当酶处理改变糖蛋白的糖基化时,糖蛋白被鉴定为来自 A498 细胞的细胞表面糖蛋白。结果揭示了将酶处理与基于 MS 的糖蛋白质组学相结合分析细胞表面糖蛋白的有效性。我们建立的方法已经证明了随着时间的推移评估治疗靶点在细胞表面的可及性并支持新靶向疗法开发的潜在应用。
更新日期:2024-11-19
中文翻译:

使用酶处理和质谱法表征细胞表面糖蛋白
细胞表面几乎所有的蛋白质都通过糖基化修饰。细胞表面糖蛋白参与各种细胞途径,例如细胞粘附、细胞间通讯和免疫反应。由于其功能重要性,细胞表面的糖蛋白通常作为潜在的治疗靶标。质谱 (MS) 的最新进展有助于表征通常位于细胞表面、分泌到细胞外环境中或在细胞内细胞器(如内质网、高尔基体和过氧化物酶体)中发现的糖蛋白。然而,细胞表面糖蛋白的选择性表征仍然具有挑战性。在这项研究中,我们对活细胞进行酶处理,然后进行基于 MS 的糖蛋白质组学分析,以评估不同处理时间点蛋白质糖基化的变化,作为鉴定细胞表面糖蛋白的方法。为了证明这种方法,在两个治疗时间间隔(2 小时和 24 小时)内用糖苷酶、唾液酸酶和 PNGase F 处理肾细胞癌细胞系 A498。当酶处理改变糖蛋白的糖基化时,糖蛋白被鉴定为来自 A498 细胞的细胞表面糖蛋白。结果揭示了将酶处理与基于 MS 的糖蛋白质组学相结合分析细胞表面糖蛋白的有效性。我们建立的方法已经证明了随着时间的推移评估治疗靶点在细胞表面的可及性并支持新靶向疗法开发的潜在应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号