当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing separation and oxidation of hydrogen sulfide through structural modification of chelated iron-based ionic liquids with organic solvents
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.seppur.2024.130591 Wenxuan Bai, Zhiping Gu, Yan Zhong, Jiang Yu
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.seppur.2024.130591 Wenxuan Bai, Zhiping Gu, Yan Zhong, Jiang Yu
|
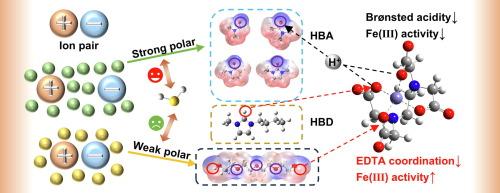
In recent years, chelated iron-based ionic liquids (BZKFeEDTA and BmimFeEDTA) have demonstrated significant promise in the oxidation and separation of H2S, attributed to their effective Fe(III) centers. However, the high viscosity of chelated iron-based ionic liquids necessitates the introduction of organic solvents to form composites for practical applications. In this study, five organic solvents (DMF, DMAC, NMP, DMI, PEG200) were incorporated into the two chelated iron-based ionic liquids to create BmimFeEDTA/solvent and BZKFeEDTA/solvent composites. The influence of solvent molecules on the ion pair molecular configurations was investigated by FT-IR and DFT calculations. Py-IR and CV were employed to assess the acidity and redox properties of the composites, while Nile red probe UV–vis spectroscopy was used to characterize the polarity of Fe-IL/solvent composites. The study demonstrates that the strong polarity of solvent molecules enhances H2S absorption, and the composites with Brønsted acidity facilitates H2S conversion. Additionally, the hydroxyl group in PEG200 significantly enhances the catalytic performance of Fe(Ⅲ) by forming hydrogen bonds with EDTA. DMI exhibits excellent properties in hydrogen sulfide adsorption and conversion due to its low LUMO-HOMO energy gap. Finally, in this paper, it is hypothesized that three interaction mechanisms regarding solvent molecules influence the catalytic activity and separation ability of chelated iron-based ionic liquids.
中文翻译:

通过使用有机溶剂对螯合铁基离子液体进行结构改性来增强硫化氢的分离和氧化
近年来,螯合铁基离子液体(BZKFeEDTA 和 BmimFeEDTA)由于其有效的 Fe(III) 中心,在 H2S 的氧化和分离方面显示出巨大的前景。然而,螯合铁基离子液体的高粘度需要引入有机溶剂来形成实际应用的复合材料。在本研究中,将 5 种有机溶剂 (DMF、DMAC、NMP、DMI、PEG200) 掺入两种螯合铁基离子液体中,制成 BmimFeEDTA/溶剂和 BZKFeEDTA/溶剂复合材料。通过 FT-IR 和 DFT 计算研究了溶剂分子对离子对分子构型的影响。Py-IR 和 CV 用于评估复合材料的酸度和氧化还原性能,而尼罗红探针紫外-可见光谱用于表征 Fe-IL/溶剂复合材料的极性。研究表明,溶剂分子的强极性增强了 H2S 的吸收,具有 Brønsted 酸性的复合材料促进了 H2S 的转化。此外,PEG200 中的羟基通过与 EDTA 形成氢键,显着增强 Fe(III.) 的催化性能。DMI 由于其低 LUMO-HOMO 能隙,在硫化氢吸附和转化方面表现出优异的性能。最后,在本文中,假设溶剂分子的三种相互作用机制会影响螯合铁基离子液体的催化活性和分离能力。
更新日期:2024-11-18
中文翻译:

通过使用有机溶剂对螯合铁基离子液体进行结构改性来增强硫化氢的分离和氧化
近年来,螯合铁基离子液体(BZKFeEDTA 和 BmimFeEDTA)由于其有效的 Fe(III) 中心,在 H2S 的氧化和分离方面显示出巨大的前景。然而,螯合铁基离子液体的高粘度需要引入有机溶剂来形成实际应用的复合材料。在本研究中,将 5 种有机溶剂 (DMF、DMAC、NMP、DMI、PEG200) 掺入两种螯合铁基离子液体中,制成 BmimFeEDTA/溶剂和 BZKFeEDTA/溶剂复合材料。通过 FT-IR 和 DFT 计算研究了溶剂分子对离子对分子构型的影响。Py-IR 和 CV 用于评估复合材料的酸度和氧化还原性能,而尼罗红探针紫外-可见光谱用于表征 Fe-IL/溶剂复合材料的极性。研究表明,溶剂分子的强极性增强了 H2S 的吸收,具有 Brønsted 酸性的复合材料促进了 H2S 的转化。此外,PEG200 中的羟基通过与 EDTA 形成氢键,显着增强 Fe(III.) 的催化性能。DMI 由于其低 LUMO-HOMO 能隙,在硫化氢吸附和转化方面表现出优异的性能。最后,在本文中,假设溶剂分子的三种相互作用机制会影响螯合铁基离子液体的催化活性和分离能力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号