Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On-Surface Synthesis of Covalently-Linked Carbaporphyrinoid-Based Low-Dimensional Polymers
Small ( IF 13.0 ) Pub Date : 2024-11-17 , DOI: 10.1002/smll.202408085
Ana Barragán 1 , Maxence Urbani 1 , Aurelio Gallardo 1 , Elena Pérez-Elvira 1 , Óscar Jover 2 , Koen Lauwaet 1 , José M Gallego 3 , Rodolfo Miranda 1 , Marco Di Giovannantonio 2 , David Écija 1, 4 , Tomás Torres 1, 5 , José I Urgel 1, 4
Small ( IF 13.0 ) Pub Date : 2024-11-17 , DOI: 10.1002/smll.202408085
Ana Barragán 1 , Maxence Urbani 1 , Aurelio Gallardo 1 , Elena Pérez-Elvira 1 , Óscar Jover 2 , Koen Lauwaet 1 , José M Gallego 3 , Rodolfo Miranda 1 , Marco Di Giovannantonio 2 , David Écija 1, 4 , Tomás Torres 1, 5 , José I Urgel 1, 4
Affiliation
|
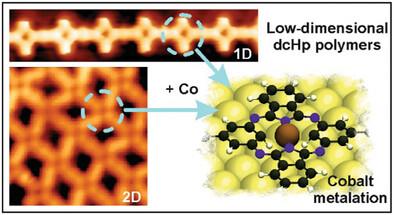
The synthesis of porphyrinoid-based low-dimensional polymers has recently attracted considerable interest in view of their intriguing electronic, optical, and catalytic properties. Here, this is introduced by the surface-assisted synthesis of two carbaporphyrinoid-based polymers of increasing dimensionality under ultrahigh-vacuum conditions. The structural and electronic characterization of the resulting polymers has been performed by scanning tunneling and non-contact atomic force microscopies, complemented by theoretical modeling. First, a carbon-carbon coupling between dicarbahemiporphyrazine precursors is achieved by thermal activation of their isopropyl substituents via a [3+3] cycloaromatization, giving rise to one-dimensional (1D) polymers. Second, the same precursor is functionalized with chlorine atoms to complement the [3+3] cycloaromatization with orthogonal dehalogenation and homocoupling, affording two-dimensional (2D) molecular nanostructures. In addition, both low-dimensional free-base porphyrinoid-based polymers are exposed to an atomic flux of cobalt atoms, giving rise to cobalt-metalated macrocycles, with the metal atoms coordinated only to the two pyrrolic nitrogens, in contrast to the typical four-fold coordination that occurs inside tetrapyrroles. This on-surface protocol renders atomically precise covalently-linked porphyrinoid polymers and provides promising model systems toward the exploration of low-coordinated metals with utility in diverse technological areas.
中文翻译:

基于共价连接的碳卟啉类化合物的表面合成
鉴于其有趣的电子、光学和催化特性,基于卟啉的低维聚合物的合成最近引起了相当大的兴趣。在这里,这是通过在超高真空条件下增加维度的两种基于碳卟啉的聚合物的表面辅助合成引入的。所得聚合物的结构和电子表征是通过扫描隧道和非接触式原子力显微镜进行的,并辅以理论建模。首先,二碳半卟啉前驱体之间的碳-碳偶联是通过 [3+3] 环芳构化对其异丙基取代基进行热活化来实现的,从而产生一维 (1D) 聚合物。其次,用氯原子对相同的前驱体进行官能化,以补充具有正交脱卤和同偶联作用的 [3+3] 环芳构化,从而得到二维 (2D) 分子纳米结构。此外,两种低维游离碱卟啉基聚合物都暴露于钴原子的原子通量中,产生钴金属化大环,金属原子仅与两个吡咯氮配位,这与四吡咯内部发生的典型四重配位形成鲜明对比。这种表面协议呈现了原子精确的共价连接的卟啉类聚合物,并为探索在不同技术领域具有实用性的低配位金属提供了有前途的模型系统。
更新日期:2024-11-17
中文翻译:

基于共价连接的碳卟啉类化合物的表面合成
鉴于其有趣的电子、光学和催化特性,基于卟啉的低维聚合物的合成最近引起了相当大的兴趣。在这里,这是通过在超高真空条件下增加维度的两种基于碳卟啉的聚合物的表面辅助合成引入的。所得聚合物的结构和电子表征是通过扫描隧道和非接触式原子力显微镜进行的,并辅以理论建模。首先,二碳半卟啉前驱体之间的碳-碳偶联是通过 [3+3] 环芳构化对其异丙基取代基进行热活化来实现的,从而产生一维 (1D) 聚合物。其次,用氯原子对相同的前驱体进行官能化,以补充具有正交脱卤和同偶联作用的 [3+3] 环芳构化,从而得到二维 (2D) 分子纳米结构。此外,两种低维游离碱卟啉基聚合物都暴露于钴原子的原子通量中,产生钴金属化大环,金属原子仅与两个吡咯氮配位,这与四吡咯内部发生的典型四重配位形成鲜明对比。这种表面协议呈现了原子精确的共价连接的卟啉类聚合物,并为探索在不同技术领域具有实用性的低配位金属提供了有前途的模型系统。






































 京公网安备 11010802027423号
京公网安备 11010802027423号