当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Dynamics of Hemoglobin in Solution and the Gas Phase Elucidated by Mass Spectrometry
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.analchem.4c01439 Julian A. Harrison, Janic Gabriel, Adam Pruška, Renato Zenobi
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.analchem.4c01439 Julian A. Harrison, Janic Gabriel, Adam Pruška, Renato Zenobi
|
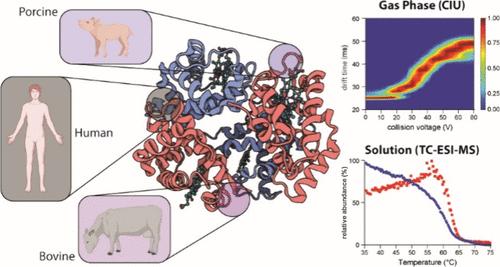
Solution and gas-phase measurements can provide valuable insights into biomolecular conformational dynamics. By comparing the data from such experiments, it is possible to elucidate the nature of the interactions governing a biomolecule’s stability. Here, we measured human, bovine, and porcine hemoglobin stability in solution and the gas phase using collision-induced dissociation, collision-induced unfolding, surface-induced dissociation, and temperature-controlled nanoelectrospray mass spectrometry. Hemoglobin dimer and tetramer stability in solution and gas phases did not correlate, likely due to differences in the composition of positive and negative amino acids on the surface of these molecules. Specifically, the absence of Lys-116 on the β-subunit makes it easier for the human hemoglobin dimer to dissociate in the gas phase. However, the presence of Lys-60 makes the subunit more rigid thus it cannot unfold to the same extent as the other hemoglobin. Hemoglobin tetramers of different origins had similar stability in the gas phase, as there was no difference in the composition of charged amino acids at the tetramer interface. These results highlight how temperature-controlled mass spectrometry and collision-induced unfolding can elucidate the structural reasons behind differences in the gas-phase and solution stability of protein complexes.
中文翻译:

溶出物中血红蛋白的构象动力学和质谱法阐明的气相
溶液和气相测量可以为生物分子构象动力学提供有价值的见解。通过比较此类实验的数据,可以阐明控制生物分子稳定性的相互作用的性质。在这里,我们使用碰撞诱导解离、碰撞诱导展开、表面诱导解离和温控纳米电喷雾质谱法测量了人、牛和猪血红蛋白在溶液和气相中的稳定性。血红蛋白二聚体和四聚体在溶液相和气相中的稳定性不相关,这可能是由于这些分子表面正氨基酸和负氨基酸组成的差异。具体来说,β 亚基上不存在 Lys-116 使得人血红蛋白二聚体更容易在气相中解离。然而,Lys-60 的存在使该亚基更加坚硬,因此它不能像其他血红蛋白那样展开。不同来源的血红蛋白四聚体在气相中具有相似的稳定性,因为四聚体界面处带电氨基酸的组成没有差异。这些结果强调了温控质谱和碰撞诱导去折叠如何阐明蛋白质复合物气相和溶液稳定性差异背后的结构原因。
更新日期:2024-11-18
中文翻译:

溶出物中血红蛋白的构象动力学和质谱法阐明的气相
溶液和气相测量可以为生物分子构象动力学提供有价值的见解。通过比较此类实验的数据,可以阐明控制生物分子稳定性的相互作用的性质。在这里,我们使用碰撞诱导解离、碰撞诱导展开、表面诱导解离和温控纳米电喷雾质谱法测量了人、牛和猪血红蛋白在溶液和气相中的稳定性。血红蛋白二聚体和四聚体在溶液相和气相中的稳定性不相关,这可能是由于这些分子表面正氨基酸和负氨基酸组成的差异。具体来说,β 亚基上不存在 Lys-116 使得人血红蛋白二聚体更容易在气相中解离。然而,Lys-60 的存在使该亚基更加坚硬,因此它不能像其他血红蛋白那样展开。不同来源的血红蛋白四聚体在气相中具有相似的稳定性,因为四聚体界面处带电氨基酸的组成没有差异。这些结果强调了温控质谱和碰撞诱导去折叠如何阐明蛋白质复合物气相和溶液稳定性差异背后的结构原因。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号