当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Cyclic 2-Aminodienes and Aminobenzofulvenes by Rhodium-Catalyzed Hydroaminative Cyclization of Diynes
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.orglett.4c03877 Hibiki Goto, Ryosuke Shiomi, Taiyoh Shimizu, Takuya Kochi, Fumitoshi Kakiuchi
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.orglett.4c03877 Hibiki Goto, Ryosuke Shiomi, Taiyoh Shimizu, Takuya Kochi, Fumitoshi Kakiuchi
|
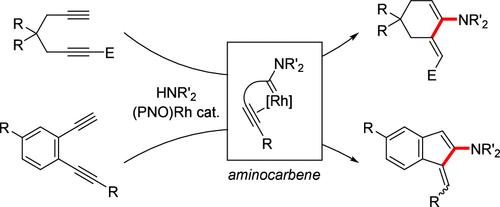
Regioselective hydroaminative cyclizations of 1,5- and 1,6-diynes via double functionalization of an alkyne carbon were achieved using a phosphine-quinolinolato (PNO) rhodium catalyst. While the reaction of 1,6-diynes with secondary amines provided cyclic 2-aminodienes, phenylene-tethered 1,5-diynes were transformed into benzofulvene derivatives. The reaction is considered to proceed via in situ construction of an aminocarbene ligand, [2 + 2] addition with an internal alkyne moiety, and isomerization to an aminodiene structure. This hydroaminative cyclization proceeds just by heating the substrate with the rhodium catalyst without adding any additive and provides a convenient route to access cyclic 2-aminodiene and aminobenzofulvene derivatives.
中文翻译:

通过铑催化的二炔氢胺基环化合成环状 2-氨基二烯和氨基苯并黄酮
使用膦-喹啉醇化 (PNO) 铑催化剂通过炔烃的双重官能化实现了 1,5-和 1,6-二炔的区域选择性氢胺环化。虽然 1,6-二炔与仲胺的反应提供了环状 2-氨基二烯,但苯基拴系的 1,5-二炔转化为苯并黄酮衍生物。该反应被认为通过原位构建氨基卡宾配体、[2 + 2] 加成内部炔烃部分以及异构化为氨基二烯结构进行。这种氢胺环化只需用铑催化剂加热基材即可进行,无需添加任何添加剂,并为获得环状 2-氨基二烯和氨基苯并黄酮衍生物提供了一种便捷的途径。
更新日期:2024-11-18
中文翻译:

通过铑催化的二炔氢胺基环化合成环状 2-氨基二烯和氨基苯并黄酮
使用膦-喹啉醇化 (PNO) 铑催化剂通过炔烃的双重官能化实现了 1,5-和 1,6-二炔的区域选择性氢胺环化。虽然 1,6-二炔与仲胺的反应提供了环状 2-氨基二烯,但苯基拴系的 1,5-二炔转化为苯并黄酮衍生物。该反应被认为通过原位构建氨基卡宾配体、[2 + 2] 加成内部炔烃部分以及异构化为氨基二烯结构进行。这种氢胺环化只需用铑催化剂加热基材即可进行,无需添加任何添加剂,并为获得环状 2-氨基二烯和氨基苯并黄酮衍生物提供了一种便捷的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号