Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insight into the Mechanism for the Cellobiose-to-Sorbitol Hydrogenation Over Diatomic Ru2/NC Catalyst
Langmuir ( IF 3.7 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.langmuir.4c03636 Ting-Hao Liu, Jin-Tao Gou, Han-Yun Min, Ming-Hui Zhang, Chang-Wei Hu, Hua-Qing Yang
Langmuir ( IF 3.7 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.langmuir.4c03636 Ting-Hao Liu, Jin-Tao Gou, Han-Yun Min, Ming-Hui Zhang, Chang-Wei Hu, Hua-Qing Yang
|
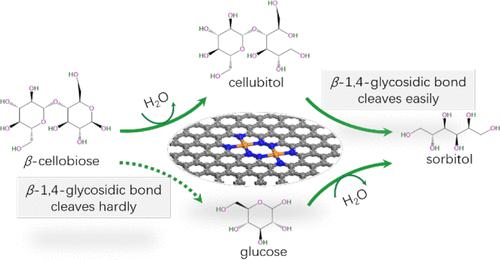
Ru/NC shows a good catalytic performance in cellobiose-to-sorbitol hydrogenation. However, the molecular origins of the selective orientation of the reaction pathway remain unclear. Here, we rationally designed the Ru2/NC catalyst, for which Ru2@N8 V4 is preferred as the model. The hydrogenation mechanisms for the hydrogenation of β-cellobiose to sorbitol employing H2 as the H-source in aqueous solution have been investigated over Ru2@N8 V4 at the GGA-PBE/DNP level. For the hydrogenation of β-cellobiose to sorbitol, the optimal reaction pathway involves the ring-opening of cellobiose with H2O as a promoter and then the hydroreduction of aldehyde group, followed by the β-1,4-glycosidic bond hydrolysis. The selective orientation of the optimal reaction pathway originates from the dissociation of H2O on Ru-sites of Ru2@N8 V4 to form Brønsted acid (Ru–H+) and Brønsted base (Ru–OH–), which collaboratively promote the ring-opening. The rate-determining steps are relative to the β-1,4-glycosidic bond cleavage, where an applicable π–π interaction between reactant molecule and Ru2@N8 V4 is of critical importance. Kinetically, the β-1,4-glycosidic bond cleavage from cellubitol is more favorable than that from β-cellobiose. For the hydrogenation of β-cellobiose to cellubitol, the first ring-opening with H2O as promoter and then hydrogenation are kinetically superior to the direct hydrogenation and ring opening. This derives from its dissociation over Ru-sites to Ru–H and Ru–OH groups. Predictably, protic solvents (HOR) are readily dissociated into Ru–H and Ru–OR at Ru-sites, which can promote the ring-opening of pyran-ring. The present research outcomes should contribute to the theoretical understanding necessary for the development of novel supported noble metal N-doped carbon catalysts for the hydrogenation of cellulose.
中文翻译:

双原子 Ru2/NC 催化剂上纤维二糖制山梨醇加氢机理的理论见解
Ru/NC 在纤维二糖制山梨醇加氢反应中表现出良好的催化性能。然而,反应途径选择性取向的分子起源仍不清楚。在这里,我们合理地设计了 Ru2/NC 催化剂,其中 Ru2@N8 V4 是首选的模型。已在 GGA-PBE/DNP 水平上研究了使用 H 2 作为水溶液中 H源β-纤维二糖加氢成山梨醇Ru2@N8氢化机制。对于 β-纤维二糖氢化为山梨醇,最佳反应途径包括以 H2O 为促进子的纤维二糖开环,然后醛基加氢还原,然后是 β-1,4-糖苷键水解。最佳反应途径的选择性取向源于 H2O 在 Ru2@N8 V4 的 Ru 位点上解离形成 Brønsted 酸 (Ru–H+) 和 Brønsted 碱 (Ru–OH–),它们共同促进开环。速率确定步骤与 β-1,4-糖苷键裂解有关,其中反应物分子和 Ru2@N8 V4 之间适用的 π-π 相互作用至关重要。在动力学上,纤维素的 β-1,4-糖苷键裂解比 β-纤维二糖的 -糖苷键裂解更有利。对于 β-纤维二糖加氢制纤维素,以 H2O 作为促进剂的第一次开环,然后加氢在动力学上优于直接加氢和开环。这源于它在 Ru 位点上解离为 Ru-H 和 Ru-OH 基团。可以预见的是,质子溶剂 (HOR) 在 Ru 位点很容易解离成 Ru-H 和 Ru-OR,这可以促进吡喃环的开环。 本研究成果应有助于开发用于纤维素加氢的新型负载贵金属 N 掺杂碳催化剂所需的理论理解。
更新日期:2024-11-18
中文翻译:

双原子 Ru2/NC 催化剂上纤维二糖制山梨醇加氢机理的理论见解
Ru/NC 在纤维二糖制山梨醇加氢反应中表现出良好的催化性能。然而,反应途径选择性取向的分子起源仍不清楚。在这里,我们合理地设计了 Ru2/NC 催化剂,其中 Ru2@N8 V4 是首选的模型。已在 GGA-PBE/DNP 水平上研究了使用 H 2 作为水溶液中 H源β-纤维二糖加氢成山梨醇Ru2@N8氢化机制。对于 β-纤维二糖氢化为山梨醇,最佳反应途径包括以 H2O 为促进子的纤维二糖开环,然后醛基加氢还原,然后是 β-1,4-糖苷键水解。最佳反应途径的选择性取向源于 H2O 在 Ru2@N8 V4 的 Ru 位点上解离形成 Brønsted 酸 (Ru–H+) 和 Brønsted 碱 (Ru–OH–),它们共同促进开环。速率确定步骤与 β-1,4-糖苷键裂解有关,其中反应物分子和 Ru2@N8 V4 之间适用的 π-π 相互作用至关重要。在动力学上,纤维素的 β-1,4-糖苷键裂解比 β-纤维二糖的 -糖苷键裂解更有利。对于 β-纤维二糖加氢制纤维素,以 H2O 作为促进剂的第一次开环,然后加氢在动力学上优于直接加氢和开环。这源于它在 Ru 位点上解离为 Ru-H 和 Ru-OH 基团。可以预见的是,质子溶剂 (HOR) 在 Ru 位点很容易解离成 Ru-H 和 Ru-OR,这可以促进吡喃环的开环。 本研究成果应有助于开发用于纤维素加氢的新型负载贵金属 N 掺杂碳催化剂所需的理论理解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号