当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trimetallic FeNiCu Atomic Clusters Supported on Carbon Matrix: Highly Active Catalysts for C3H6–SCR of NO
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acsami.4c11698 Shuying Ning, Nini Wen, Bingtao Zhao, Muhammad Kashif, Philippe M. Heynderickx, Yaxin Su
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acsami.4c11698 Shuying Ning, Nini Wen, Bingtao Zhao, Muhammad Kashif, Philippe M. Heynderickx, Yaxin Su
|
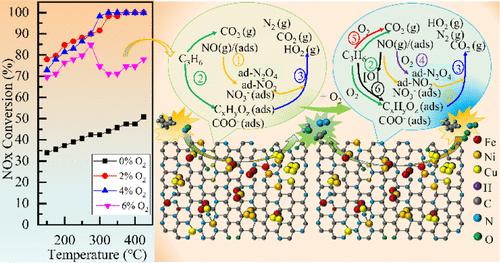
C3H6–SCR denitrification technology faces catalyst deactivation problems and low catalytic performance at medium-low temperatures. This study utilized the intermetallic synergies to prepare atomic cluster catalysts (FeNiCu/NC) by anchoring Fe–Ni–Cu on a carbon matrix to enhance the C3H6–SCR performance at medium-low temperatures. The synergistic effect of the Fe–Ni–Cu is reflected in the differences in the physicochemical properties of the catalysts, which is proved by several characterization techniques. Results showed that the FeNiCu/NC catalyst had a larger surface area (541.4 m2/g) and there were no metal oxides on the surface of the catalyst but abundant defective sites that anchored Fe/Ni/Cu atoms through N atoms to form M–Nx active sites and atomic clusters. The hollow carbon morphology provides sufficient active sites for C3H6–SCR. The coordination environments of active sites were M–Nx–C, Fe2/FeCu2/FeNi2, Ni3/NiFe/NiCu2, and Cu4/CuFe2/CuNi2, where the synergistic action of trimetal leads to the presence of Fe–Ni–Cu–Nx–C. The synergistic action of the Fe–Ni–Cu significantly improved the C3H6–SCR performance at medium-low temperatures. The FeNiCu/NC exhibited an 81% NO conversion at 150 °C under 2% O2, 15% and 20% higher than FeNi/NC and FeCu/NC catalysts, respectively. Even at 4% O2, the FeNiCu/NC catalyst was active to remove 78% NO and achieve a 93% N2 selectivity at 150 °C and maintained a 100% NO conversion at 300–425 °C. The DRIFTS results demonstrated that NO and C3H6 could combine with active O at metal cluster, M–Nx, or defective oxygen sites to produce various intermediate species, wherein acetates and nitrates were the main active intermediates. Based on the DRIFTS results, a reaction pathway for C3H6–SCR over the FeNiCu/NC catalyst was proposed.
中文翻译:

碳基体上负载的三金属 FeNiCu 原子团簇:用于 C3H6–SCR 为 NO 的高活性催化剂
C3H6-SCR 脱硝技术在中低温下面临催化剂失活和低催化性能问题。本研究利用金属间化合物协同作用制备原子簇催化剂 (FeNiCu/NC),通过将 Fe-Ni-Cu 锚定在碳基体上,以增强 C3H 6-SCR 在中低温下的性能。Fe-Ni-Cu 的协同效应反映在催化剂物理化学性质的差异上,这可以通过多种表征技术得到证明。结果表明,FeNiCu/NC 催化剂具有较大的表面积 (541.4 m2/g),催化剂表面没有金属氧化物,但有丰富的缺陷位点,这些缺陷位点将 Fe/Ni/Cu 原子通过 N 原子锚定,形成 M–Nx 活性位点和原子团簇。空心碳形态为 C3H 6-SCR 提供了足够的活性位点。活性位点的配位环境为 M-Nx-C、Fe2/FeCu2/FeNi2、Ni3/NiFe/NiCu2 和 Cu4/CuFe2/CuNi2,其中三金属的协同作用导致 Fe-Ni-Cu-Nx-C 的存在。Fe-Ni-Cu 的协同作用显著提高了 C3H 6-SCR 在中低温下的性能。在 150 °C 下,在 2% O2 下,FeNiCu/NC 的 NO 转化率为 81%,分别比 FeNi/NC 和 FeCu/NC 催化剂高 15% 和 20%。即使在 4% O2 下,FeNiCu/NC 催化剂在 150 °C 下也能活性去除 78% 的 NO 并实现 93% 的 N2 选择性,并在 300–425 °C 下保持 100% 的 NO 转化率。 DRIFTS 结果表明,NO 和 C3H6 可以在金属簇、M–Nx 或缺陷氧位点与活性 O 结合,产生各种中间物种,其中乙酸盐和硝酸盐是主要的活性中间体。基于 DRIFTS 结果,提出了 FeNiCu/NC 催化剂上 C3H6-SCR 的反应途径。
更新日期:2024-11-18
中文翻译:

碳基体上负载的三金属 FeNiCu 原子团簇:用于 C3H6–SCR 为 NO 的高活性催化剂
C3H6-SCR 脱硝技术在中低温下面临催化剂失活和低催化性能问题。本研究利用金属间化合物协同作用制备原子簇催化剂 (FeNiCu/NC),通过将 Fe-Ni-Cu 锚定在碳基体上,以增强 C3H 6-SCR 在中低温下的性能。Fe-Ni-Cu 的协同效应反映在催化剂物理化学性质的差异上,这可以通过多种表征技术得到证明。结果表明,FeNiCu/NC 催化剂具有较大的表面积 (541.4 m2/g),催化剂表面没有金属氧化物,但有丰富的缺陷位点,这些缺陷位点将 Fe/Ni/Cu 原子通过 N 原子锚定,形成 M–Nx 活性位点和原子团簇。空心碳形态为 C3H 6-SCR 提供了足够的活性位点。活性位点的配位环境为 M-Nx-C、Fe2/FeCu2/FeNi2、Ni3/NiFe/NiCu2 和 Cu4/CuFe2/CuNi2,其中三金属的协同作用导致 Fe-Ni-Cu-Nx-C 的存在。Fe-Ni-Cu 的协同作用显著提高了 C3H 6-SCR 在中低温下的性能。在 150 °C 下,在 2% O2 下,FeNiCu/NC 的 NO 转化率为 81%,分别比 FeNi/NC 和 FeCu/NC 催化剂高 15% 和 20%。即使在 4% O2 下,FeNiCu/NC 催化剂在 150 °C 下也能活性去除 78% 的 NO 并实现 93% 的 N2 选择性,并在 300–425 °C 下保持 100% 的 NO 转化率。 DRIFTS 结果表明,NO 和 C3H6 可以在金属簇、M–Nx 或缺陷氧位点与活性 O 结合,产生各种中间物种,其中乙酸盐和硝酸盐是主要的活性中间体。基于 DRIFTS 结果,提出了 FeNiCu/NC 催化剂上 C3H6-SCR 的反应途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号