Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the aminopeptidase ERAP enhances antitumor immunity by disrupting the NKG2A-HLA-E inhibitory checkpoint
Immunity ( IF 25.5 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.immuni.2024.10.013 Hsiao-Wei Tsao, Seth Anderson, Kenneth J. Finn, Jonathan J. Perera, Lomax F. Pass, Emily M. Schneider, Aiping Jiang, Rachel Fetterman, Cun Lan Chuong, Kaiya Kozuma, Marcia M. Stickler, Marc Creixell, Susan Klaeger, Kshiti Meera Phulphagar, Suzanna Rachimi, Eva K. Verzani, Niclas Olsson, Juan Dubrot, Matthew F. Pech, Whitney Silkworth, Robert T. Manguso
Immunity ( IF 25.5 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.immuni.2024.10.013 Hsiao-Wei Tsao, Seth Anderson, Kenneth J. Finn, Jonathan J. Perera, Lomax F. Pass, Emily M. Schneider, Aiping Jiang, Rachel Fetterman, Cun Lan Chuong, Kaiya Kozuma, Marcia M. Stickler, Marc Creixell, Susan Klaeger, Kshiti Meera Phulphagar, Suzanna Rachimi, Eva K. Verzani, Niclas Olsson, Juan Dubrot, Matthew F. Pech, Whitney Silkworth, Robert T. Manguso
|
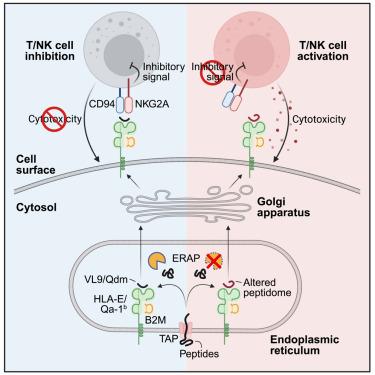
The aminopeptidase, endoplasmic reticulum aminopeptidase 1 (ERAP1), trims peptides for loading into major histocompatibility complex class I (MHC class I), and loss of this activity has broad effects on the MHC class I peptidome. Here, we investigated the impact of targeting ERAP1 in immune checkpoint blockade (ICB), as MHC class I interactions mediate both activating and inhibitory functions in antitumor immunity. Loss of ERAP sensitized mouse tumor models to ICB, and this sensitivity depended on CD8+ T cells and natural killer (NK) cells. In vivo suppression screens revealed that Erap1 deletion inactivated the inhibitory NKG2A-HLA-E checkpoint, which requires presentation of a restricted set of invariant epitopes (VL9) on HLA-E. Loss of ERAP altered the HLA-E peptidome, preventing NKG2A engagement. In humans, ERAP1 and ERAP2 showed functional redundancy for the processing and presentation of VL9, and loss of both inactivated the NKG2A checkpoint in cancer cells. Thus, loss of ERAP phenocopies the inhibition of the NKG2A-HLA-E pathway and represents an attractive approach to inhibit this critical checkpoint.
中文翻译:

靶向氨肽酶 ERAP 通过破坏 NKG2A-HLA-E 抑制检查点来增强抗肿瘤免疫力
氨肽酶,内质网氨肽酶 1 (ERAP1),修剪肽以加载到主要组织相容性复合物 I 类(MHC I 类)中,这种活性的丧失对 MHC I 类肽组有广泛的影响。在这里,我们研究了靶向 ERAP1 在免疫检查点阻断 (ICB) 中的影响,因为 MHC I 类相互作用介导抗肿瘤免疫中的激活和抑制功能。ERAP 的缺失使小鼠肿瘤模型对 ICB 敏感,这种敏感性取决于 CD8+ T 细胞和自然杀伤 (NK) 细胞。 体内抑制筛选显示,Erap1 缺失使抑制性 NKG2A-HLA-E 检查点失活,这需要在 HLA-E 上呈现一组受限的不变表位 (VL9)。ERAP 缺失改变了 HLA-E 肽组,阻止了 NKG2A 参与。在人类中,ERAP1 和 ERAP2 在 VL9 的加工和呈递方面显示出功能冗余,而两者的缺失会使癌细胞中的 NKG2A 检查点失活。因此,ERAP 表型的缺失复制了 NKG2A-HLA-E 通路的抑制,代表了抑制这一关键检查点的一种有吸引力的方法。
更新日期:2024-11-18
中文翻译:

靶向氨肽酶 ERAP 通过破坏 NKG2A-HLA-E 抑制检查点来增强抗肿瘤免疫力
氨肽酶,内质网氨肽酶 1 (ERAP1),修剪肽以加载到主要组织相容性复合物 I 类(MHC I 类)中,这种活性的丧失对 MHC I 类肽组有广泛的影响。在这里,我们研究了靶向 ERAP1 在免疫检查点阻断 (ICB) 中的影响,因为 MHC I 类相互作用介导抗肿瘤免疫中的激活和抑制功能。ERAP 的缺失使小鼠肿瘤模型对 ICB 敏感,这种敏感性取决于 CD8+ T 细胞和自然杀伤 (NK) 细胞。 体内抑制筛选显示,Erap1 缺失使抑制性 NKG2A-HLA-E 检查点失活,这需要在 HLA-E 上呈现一组受限的不变表位 (VL9)。ERAP 缺失改变了 HLA-E 肽组,阻止了 NKG2A 参与。在人类中,ERAP1 和 ERAP2 在 VL9 的加工和呈递方面显示出功能冗余,而两者的缺失会使癌细胞中的 NKG2A 检查点失活。因此,ERAP 表型的缺失复制了 NKG2A-HLA-E 通路的抑制,代表了抑制这一关键检查点的一种有吸引力的方法。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号