当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deoxygenative alcohol–nucleophile coupling via carbocations
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.checat.2024.101187 Léa Thai-Savard, Jason R. Zbieg, Jack A. Terrett
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.checat.2024.101187 Léa Thai-Savard, Jason R. Zbieg, Jack A. Terrett
|
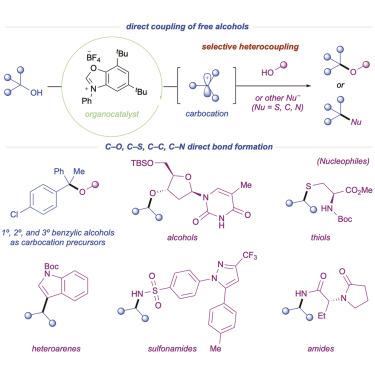
The direct employment of widely available alcohol feedstocks as synthons in nucleophilic couplings is a long-standing objective within the synthetic community. Traditional methods utilizing alcohols require the preactivation of one coupling partner due to the inherent mismatched electronics for C–O bond formation. Here, free alcohols are leveraged as carbocation precursors via in situ activation, reversing their traditional nucleophilic behavior and avoiding the need for prefunctionalization. The direct catalytic deoxygenative coupling of alcohols toward selective C–O heterocoupling is described. Mechanistic studies support the intermediacy of a discrete carbocation, which can be intercepted by a diverse array of simple nucleophiles. Application of this protocol toward natural products and complex active pharmaceutical ingredients is also demonstrated. The compatibility toward a large breadth of nucleophiles enables the construction of C–O, C–S, C–C, and C–N bonds in a single step, showcasing the broad applicability of this alcohol activation platform.
中文翻译:

通过碳阳离子进行脱氧醇-亲核偶联
在亲核偶联中直接使用广泛可用的醇原料作为合成子是合成生物界的一个长期目标。使用醇类的传统方法需要预活化一个偶联伴侣,因为 C-O 键形成存在固有的错配电子元件。在这里,游离醇通过原位活化被用作碳阳离子前体,逆转了它们传统的亲核行为,避免了前官能团化的需要。描述了醇对选择性 C-O 异质偶联的直接催化脱氧偶联。机理研究支持离散碳阳离子的中介性,它可以被各种简单的亲核试剂拦截。还证明了该方案对天然产物和复杂活性药物成分的应用。与大量亲核试剂的相容性使其能够一步构建 C-O、C-S、C-C 和 C-N 键,展示了该醇活化平台的广泛适用性。
更新日期:2024-11-18
中文翻译:

通过碳阳离子进行脱氧醇-亲核偶联
在亲核偶联中直接使用广泛可用的醇原料作为合成子是合成生物界的一个长期目标。使用醇类的传统方法需要预活化一个偶联伴侣,因为 C-O 键形成存在固有的错配电子元件。在这里,游离醇通过原位活化被用作碳阳离子前体,逆转了它们传统的亲核行为,避免了前官能团化的需要。描述了醇对选择性 C-O 异质偶联的直接催化脱氧偶联。机理研究支持离散碳阳离子的中介性,它可以被各种简单的亲核试剂拦截。还证明了该方案对天然产物和复杂活性药物成分的应用。与大量亲核试剂的相容性使其能够一步构建 C-O、C-S、C-C 和 C-N 键,展示了该醇活化平台的广泛适用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号