当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton Relays in Molecular Catalysis for Hydrogen Evolution and Oxidation: Lessons From the Mimicry of Hydrogenases and Electrochemical Kinetic Analyses
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-11-18 , DOI: 10.1002/anie.202413910 Matthieu Haake, Bertrand Reuillard, Murielle Chavarot‐Kerlidou, Cyrille Costentin, Vincent Artero
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-11-18 , DOI: 10.1002/anie.202413910 Matthieu Haake, Bertrand Reuillard, Murielle Chavarot‐Kerlidou, Cyrille Costentin, Vincent Artero
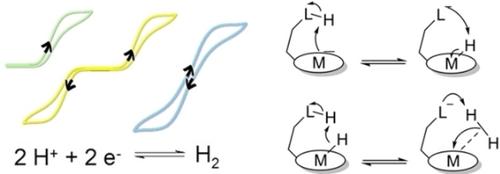
|
The active sites of metalloenzymes involved in small molecules activation often contain pendant bases that act as proton relay promoting proton‐coupled electron‐transfer processes. Here we focus on hydrogenases and on the reactions they catalyze, i. e. the hydrogen evolution and oxidation reactions. After a short description of these enzymes, we review some of the various biomimetic and bioinspired molecular systems that contain proton relays. We then provide the formal electrochemical framework required to decipher the key role of such proton relay to enhance catalysis in a single direction and discuss the few systems active for H2 evolution for which quantitative kinetic data are available. We finally highlight key parameters required to reach bidirectional catalysis (both hydrogen evolution and hydrogen oxidation catalyzed) and then transition to reversible catalysis (both reactions catalyzed in a narrow potential range) as well as illustrate these features on few systems from the literature.
中文翻译:

分子催化析氢和氧化中的质子中继:氢化酶模拟和电化学动力学分析的经验教训
参与小分子活化的金属酶的活性位点通常包含悬垂碱基,这些碱基充当质子中继,促进质子耦合电子转移过程。在这里,我们专注于氢化酶及其催化的反应,即析氢和氧化反应。在对这些酶进行简短描述之后,我们回顾了一些包含质子中继的各种仿生和仿生分子系统。然后,我们提供了破译这种质子中继在增强单一方向催化方面的关键作用所需的正式电化学框架,并讨论了少数几个对 H2 释放有活性的系统,这些系统有定量动力学数据。最后,我们强调了达到双向催化(析氢和氢氧化催化)然后过渡到可逆催化(两种反应都在狭窄的电位范围内催化)所需的关键参数,并在文献中的少数系统上说明了这些特征。
更新日期:2024-11-18
中文翻译:

分子催化析氢和氧化中的质子中继:氢化酶模拟和电化学动力学分析的经验教训
参与小分子活化的金属酶的活性位点通常包含悬垂碱基,这些碱基充当质子中继,促进质子耦合电子转移过程。在这里,我们专注于氢化酶及其催化的反应,即析氢和氧化反应。在对这些酶进行简短描述之后,我们回顾了一些包含质子中继的各种仿生和仿生分子系统。然后,我们提供了破译这种质子中继在增强单一方向催化方面的关键作用所需的正式电化学框架,并讨论了少数几个对 H2 释放有活性的系统,这些系统有定量动力学数据。最后,我们强调了达到双向催化(析氢和氢氧化催化)然后过渡到可逆催化(两种反应都在狭窄的电位范围内催化)所需的关键参数,并在文献中的少数系统上说明了这些特征。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号