当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Highly Potent Os@Au-TPA Coordination Structure-Based Sonosensitizer for Tumor Sono-Immunotherapies
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-11-18 , DOI: 10.1002/adfm.202412564 Pengfei Xie, Xiao Rong, Xuelian Qin, Min Li, Yan Zuo, Bingjie Liu, Sujiao Cao, Jie Yang, Li Qiu
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-11-18 , DOI: 10.1002/adfm.202412564 Pengfei Xie, Xiao Rong, Xuelian Qin, Min Li, Yan Zuo, Bingjie Liu, Sujiao Cao, Jie Yang, Li Qiu
|
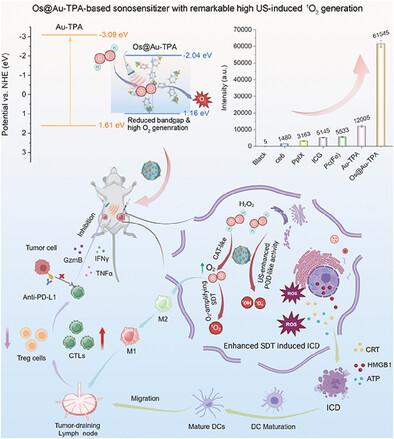
Ultrasound (US) becomes an appealing modality for stimulating or amplifying immune responses during cancer therapy, which is also termed sono-immunotherapy. However, the clinical prospect has not been fully realized due to the scarcity of efficient sonosensitizers. Herein, for the first time a novel Os-doped Au-tri(pyridin-4-yl) amine coordination structure (Os@Au-TPA)-based sonosensitizer is originally designed and synthesized for sono-immunotherapy of breast-metastasized tumors. Impressively, Os@Au-TPA shows much higher US-mediated 1O2-producing activity than Au-TPA as well as the other traditional sonosensitizers, for example, ≈41.6 folds to ce6, 19.5 times to Protoporphyrin IX (PpIX), 12.0 to Indocyanine Green (ICG), and 11.1 to Iron phthalocyanine (Pc(Fe)). The Os@Au-TPA can not only generate abundant ROS upon US irradiation to implement sonodynamic therapy (SDT), stimulating cell apoptosis and further immunogenic cell death, but can also generate O2 to alleviate hypoxia to promote the polarization of M2 to M1 macrophages to enhance tumor immunogenicity. As a result, when combined with PD-L1 antibody, it remodels the immunosuppressive tumor microenvironment, achieves concurrent sonodynamic-triggered immune activation, and eradicates both the original and distant-metastasized tumors efficiently. This work not only provides a new strategy to construct potent sonosensitizers from pyridine-metal coordination structures but also proves that sonosensitizers with high performance are crucial in boosting cancer sono-immunotherapy.
中文翻译:

一种用于肿瘤声Os@Au免疫疗法的高效 -TPA 配位结构增敏剂
超声 (US) 成为癌症治疗期间刺激或放大免疫反应的一种有吸引力的方式,也称为声波免疫疗法。然而,由于高效声敏剂的稀缺,临床前景尚未完全实现。在此,首次设计和合成了一种新型的基于 Os掺杂的 Au-三(吡啶-4-基)胺配位结构 (Os@Au-TPA) 的声敏剂,用于乳腺转移性肿瘤的声波免疫治疗。令人印象深刻的是,Os@Au-TPA 显示出比 Au-TPA 以及其他传统声敏剂高得多的 US 介导的 1O2 产生活性,例如≈,ce6 是 41.6 倍,是原卟啉 IX (PpIX) 的 19.5 倍,吲哚菁绿 (ICG) 是 12.0 倍,酞菁铁 (Pc(Fe)) 是 11.1 倍。Os@Au-TPA 不仅可以在 US 照射下产生丰富的 ROS 以实施声动力学疗法 (SDT),刺激细胞凋亡和进一步的免疫原性细胞死亡,还可以产生 O2 以缓解缺氧,促进 M2 向 M1 巨噬细胞的极化,从而增强肿瘤免疫原性。因此,当与 PD-L1 抗体结合时,它重塑免疫抑制性肿瘤微环境,实现同时声动力学触发的免疫激活,并有效根除原始和远处转移的肿瘤。这项工作不仅提供了一种从吡啶-金属配位结构构建有效声敏剂的新策略,而且还证明了高性能的声敏剂在促进癌症声波免疫治疗中至关重要。
更新日期:2024-11-18
中文翻译:

一种用于肿瘤声Os@Au免疫疗法的高效 -TPA 配位结构增敏剂
超声 (US) 成为癌症治疗期间刺激或放大免疫反应的一种有吸引力的方式,也称为声波免疫疗法。然而,由于高效声敏剂的稀缺,临床前景尚未完全实现。在此,首次设计和合成了一种新型的基于 Os掺杂的 Au-三(吡啶-4-基)胺配位结构 (Os@Au-TPA) 的声敏剂,用于乳腺转移性肿瘤的声波免疫治疗。令人印象深刻的是,Os@Au-TPA 显示出比 Au-TPA 以及其他传统声敏剂高得多的 US 介导的 1O2 产生活性,例如≈,ce6 是 41.6 倍,是原卟啉 IX (PpIX) 的 19.5 倍,吲哚菁绿 (ICG) 是 12.0 倍,酞菁铁 (Pc(Fe)) 是 11.1 倍。Os@Au-TPA 不仅可以在 US 照射下产生丰富的 ROS 以实施声动力学疗法 (SDT),刺激细胞凋亡和进一步的免疫原性细胞死亡,还可以产生 O2 以缓解缺氧,促进 M2 向 M1 巨噬细胞的极化,从而增强肿瘤免疫原性。因此,当与 PD-L1 抗体结合时,它重塑免疫抑制性肿瘤微环境,实现同时声动力学触发的免疫激活,并有效根除原始和远处转移的肿瘤。这项工作不仅提供了一种从吡啶-金属配位结构构建有效声敏剂的新策略,而且还证明了高性能的声敏剂在促进癌症声波免疫治疗中至关重要。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号