当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An ultra-fast reaction process for recycling lithium ion batteries via galvanic cell interaction
Chemical Science ( IF 7.6 ) Pub Date : 2024-11-18 , DOI: 10.1039/d4sc06076h Long Ye, Zhilong Xu, Haiqiang Gong, Zhiming Xiao, Bao Zhang, Lei Ming, Xing Ou
Chemical Science ( IF 7.6 ) Pub Date : 2024-11-18 , DOI: 10.1039/d4sc06076h Long Ye, Zhilong Xu, Haiqiang Gong, Zhiming Xiao, Bao Zhang, Lei Ming, Xing Ou
|
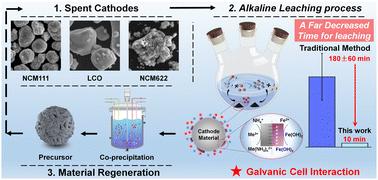
The efficient realization of a closed-loop process is an ultimate goal for reusing spent lithium-ion batteries (LIBs), yet the complicated recycling processes of leaching and purification in an acid atmosphere are totally different compared with the regeneration method of the cathode precursor in alkali solution, inevitably resulting in the redundant consumption of acid/ammonia solutions and increased burden for a green environment. Herein, considering the advantages of selective extraction and similar chemical surroundings for recovery and regeneration, ammonia-leaching has been proposed to achieve short-process closed-loop recycling with effective impurity removal. Particularly, benefiting from the galvanic cell interaction, the sluggish reaction rate and relatively harsh reaction conditions of ammonia-leaching are well addressed. High leaching efficiency can be achieved within 10 min, where nearly 80% valuable metals are extracted in the initial 1 min. Notably, this leaching solution, after purification, can be used to directly synthesize the cathode precursor through the commercial alkali co-precipitation method. This process is superior to the acid leaching system, which requires the use of acid–base solutions back and forth to adjust pH for metal extraction and material regeneration. Compared to the traditional solid-to-liquid reaction with a shrinking core model, the solid-to-solid reaction with galvanic cell interaction substantially addresses the inherent issue of sluggish leaching efficiency, exhibiting much stronger competitiveness in the leaching rate and environment cost. Thus, it provides prospects to achieve large-scale recycling and regeneration of spent LIBs simultaneously in the whole-process alkali-atmosphere.
中文翻译:

一种通过原电池相互作用回收锂离子电池的超快速反应工艺
高效实现闭环过程是废旧锂离子电池 (LIB) 再利用的最终目标,但在酸性气氛中浸出和净化的复杂回收过程与碱溶液中正极前驱体的再生方法完全不同,不可避免地导致酸/氨溶液的冗余消耗和对绿色环境的负担增加。在此,考虑到选择性提取和相似化学环境对回收和再生的优势,提出了氨浸出法,以实现短过程闭环回收和有效除杂。特别是,受益于原电池相互作用,氨浸出反应速率缓慢和相对恶劣的反应条件得到了很好的解决。可在 10 分钟内实现高浸出效率,其中近 80% 的有价金属在最初的 1 分钟内被提取出来。值得注意的是,这种浸出液经过纯化后,可用于通过商业碱共沉淀法直接合成阴极前驱体。该工艺优于酸浸系统,后者需要来回使用酸碱溶液来调节 pH 值以进行金属提取和材料再生。与传统的缩核固-液反应模型相比,原电池相互作用的固-固反应实质上解决了浸出效率低的固有问题,在浸出速率和环境成本方面表现出更强的竞争力。因此,它为在全过程碱气氛中同时实现废锂离子电池的大规模回收和再生提供了前景。
更新日期:2024-11-18
中文翻译:

一种通过原电池相互作用回收锂离子电池的超快速反应工艺
高效实现闭环过程是废旧锂离子电池 (LIB) 再利用的最终目标,但在酸性气氛中浸出和净化的复杂回收过程与碱溶液中正极前驱体的再生方法完全不同,不可避免地导致酸/氨溶液的冗余消耗和对绿色环境的负担增加。在此,考虑到选择性提取和相似化学环境对回收和再生的优势,提出了氨浸出法,以实现短过程闭环回收和有效除杂。特别是,受益于原电池相互作用,氨浸出反应速率缓慢和相对恶劣的反应条件得到了很好的解决。可在 10 分钟内实现高浸出效率,其中近 80% 的有价金属在最初的 1 分钟内被提取出来。值得注意的是,这种浸出液经过纯化后,可用于通过商业碱共沉淀法直接合成阴极前驱体。该工艺优于酸浸系统,后者需要来回使用酸碱溶液来调节 pH 值以进行金属提取和材料再生。与传统的缩核固-液反应模型相比,原电池相互作用的固-固反应实质上解决了浸出效率低的固有问题,在浸出速率和环境成本方面表现出更强的竞争力。因此,它为在全过程碱气氛中同时实现废锂离子电池的大规模回收和再生提供了前景。































 京公网安备 11010802027423号
京公网安备 11010802027423号