当前位置:
X-MOL 学术
›
J. Materiomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
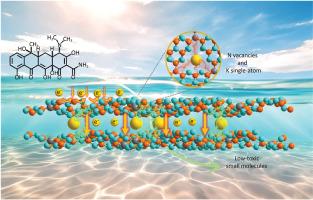
Dual-site engineering of N vacancies and K single-atoms in C3N4: Enabling spatial charge transfer channels for photocatalysis
Journal of Materiomics ( IF 8.4 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.jmat.2024.100969 Xiao Xu, Yao Xiao, Xuelian Xu, Sónia A.C. Carabineiro, Junjiang Zhu
Journal of Materiomics ( IF 8.4 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.jmat.2024.100969 Xiao Xu, Yao Xiao, Xuelian Xu, Sónia A.C. Carabineiro, Junjiang Zhu
|
Graphitic carbon nitride (C3N4) is a promising photocatalyst due to its suitable band gap and polymer properties, but its efficiency is limited by the poor separation of photoinduced electron/hole (e–/h+) pairs. To address this issue, we propose creating N vacancies within the layers and bridging K single-atoms between the C3N4 layers through the self-assembly of potassium citrate and melamine–urea monomers. The introduction of N vacancies disrupts the symmetry of C3N4, promoting electron transfer along the delocalized π-conjugated network, while the presence of K atoms provides channels for electron transfer between the layers by forming N K
K N bridges, thereby leading to significant enhancement in the separation and transfer of e–/h+ pairs across spatial dimension. As expected, the co-modified C3N4, with N vacancies and K single-atoms (designated as CN-K-VN), exhibits excellent photocatalytic performance, with reaction rate constant of 9.69 × 10−2 min−1 (7.39 × 10−2 min−1 in real water environment) for tetracycline, achieving 80% degradation of tetracycline within 20 min. The reaction mechanism, as well as the toxicity of the degradation intermediates, is deeply discussed. This study provides a strategy to enhance the spatial separation of electrons for photocatalyst, highlighting its significance role in photocatalysis.
N bridges, thereby leading to significant enhancement in the separation and transfer of e–/h+ pairs across spatial dimension. As expected, the co-modified C3N4, with N vacancies and K single-atoms (designated as CN-K-VN), exhibits excellent photocatalytic performance, with reaction rate constant of 9.69 × 10−2 min−1 (7.39 × 10−2 min−1 in real water environment) for tetracycline, achieving 80% degradation of tetracycline within 20 min. The reaction mechanism, as well as the toxicity of the degradation intermediates, is deeply discussed. This study provides a strategy to enhance the spatial separation of electrons for photocatalyst, highlighting its significance role in photocatalysis.
中文翻译:

C3N4 中 N 空位和 K 单原子的双位工程:为光催化实现空间电荷转移通道
石墨氮化碳 (C3N4) 由于其合适的带隙和聚合物特性而是一种很有前途的光催化剂,但其效率受到光诱导电子/空穴 (e–/h+) 对分离不良的限制。为了解决这个问题,我们建议在层内创建 N 空位,并通过柠檬酸钾和三聚氰胺-尿素单体的自组装在 C3N4 层之间桥接 K 单原子。N 空位的引入破坏了 C3N4 的对称性,促进了沿离域 π 共轭网络的电子转移,而 K 原子的存在通过形成 NK
 N 桥为层间的电子转移提供了通道,从而显着增强了 e–/h+ 对在空间维度上的分离和转移。正如预期的那样,共修饰的 C3N4 具有 N 空位和 K 单原子(指定为 CN-K-VN),表现出优异的光催化性能,对四环素的反应速率常数为 9.69 × 10-2 min-1(在实际水环境中为 7.39 × 10-2 min-1),在 20 分钟内实现 80% 的四环素降解。深入讨论了反应机理以及降解中间体的毒性。本研究提供了一种增强光催化剂电子空间分离的策略,突出了其在光催化中的重要作用。
N 桥为层间的电子转移提供了通道,从而显着增强了 e–/h+ 对在空间维度上的分离和转移。正如预期的那样,共修饰的 C3N4 具有 N 空位和 K 单原子(指定为 CN-K-VN),表现出优异的光催化性能,对四环素的反应速率常数为 9.69 × 10-2 min-1(在实际水环境中为 7.39 × 10-2 min-1),在 20 分钟内实现 80% 的四环素降解。深入讨论了反应机理以及降解中间体的毒性。本研究提供了一种增强光催化剂电子空间分离的策略,突出了其在光催化中的重要作用。
更新日期:2024-11-17
 K
K N bridges, thereby leading to significant enhancement in the separation and transfer of e–/h+ pairs across spatial dimension. As expected, the co-modified C3N4, with N vacancies and K single-atoms (designated as CN-K-VN), exhibits excellent photocatalytic performance, with reaction rate constant of 9.69 × 10−2 min−1 (7.39 × 10−2 min−1 in real water environment) for tetracycline, achieving 80% degradation of tetracycline within 20 min. The reaction mechanism, as well as the toxicity of the degradation intermediates, is deeply discussed. This study provides a strategy to enhance the spatial separation of electrons for photocatalyst, highlighting its significance role in photocatalysis.
N bridges, thereby leading to significant enhancement in the separation and transfer of e–/h+ pairs across spatial dimension. As expected, the co-modified C3N4, with N vacancies and K single-atoms (designated as CN-K-VN), exhibits excellent photocatalytic performance, with reaction rate constant of 9.69 × 10−2 min−1 (7.39 × 10−2 min−1 in real water environment) for tetracycline, achieving 80% degradation of tetracycline within 20 min. The reaction mechanism, as well as the toxicity of the degradation intermediates, is deeply discussed. This study provides a strategy to enhance the spatial separation of electrons for photocatalyst, highlighting its significance role in photocatalysis.
中文翻译:

C3N4 中 N 空位和 K 单原子的双位工程:为光催化实现空间电荷转移通道
石墨氮化碳 (C3N4) 由于其合适的带隙和聚合物特性而是一种很有前途的光催化剂,但其效率受到光诱导电子/空穴 (e–/h+) 对分离不良的限制。为了解决这个问题,我们建议在层内创建 N 空位,并通过柠檬酸钾和三聚氰胺-尿素单体的自组装在 C3N4 层之间桥接 K 单原子。N 空位的引入破坏了 C3N4 的对称性,促进了沿离域 π 共轭网络的电子转移,而 K 原子的存在通过形成 NK































 京公网安备 11010802027423号
京公网安备 11010802027423号