当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia Synthesis with Visible Light and Quantum Dots
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-17 , DOI: 10.1021/jacs.4c06713 Vanshika Jain, Shreya Tyagi, Pradyut Roy, Pramod P. Pillai
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-17 , DOI: 10.1021/jacs.4c06713 Vanshika Jain, Shreya Tyagi, Pradyut Roy, Pramod P. Pillai
|
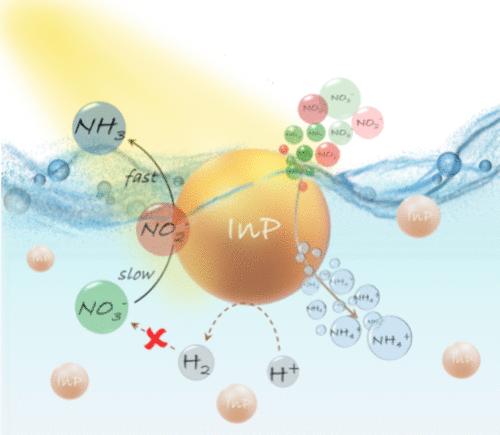
Light-assisted synthesis of ammonia from nitrate and nitrite sources is a sustainable approach to reduce the burden of the energy-intensive Haber-Bosch process. However, poor selectivity and the need for UV-active photocatalysts are the current bottlenecks in the synthesis of ammonia from nitrate and nitrite sources. Herein, we introduce selective visible-light-driven ammonia production from nitrate and nitrite ions with indium phosphide quantum dots (InP QDs) as the photocatalyst. The presence of catalytic indium sites and microenvironment modulation through an interplay of catalyst-reactant interactions resulted in efficient and selective ammonia formation under visible light. Ammonia was produced in an attractive yield of ∼94% in both aqueous and gaseous phases within 2 h of visible-light irradiation at room temperature. A decent formation of ammonia was observed under sunlight as well, strengthening the translational prospects of InP QD photocatalysts. Mechanistic investigations ascertained a negligible role of competing hydrogen evolution in direct nitrate reduction, confirming the active participation of photoexcited charge carriers from InP QDs in the ammonia synthesis. Kinetic studies revealed the energetically challenging nitrate-to-nitrite conversion as the rate-determining step, with subsequent reactions proceeding with ∼100% conversion to yield ammonia. A series of experiments concluded that water is the proton source in the InP QD-photocatalyzed synthesis of ammonia. Our study shows the impact of the rationally designed core and surface of InP QD-based photocatalysts in developing sustainable routes to produce ammonia beyond the Haber-Bosch process.
中文翻译:

用可见光和量子点合成氨
从硝酸盐和亚硝酸盐来源光辅助合成氨是一种可持续的方法,可以减轻能源密集型 Haber-Bosch 工艺的负担。然而,选择性差和对紫外线活性光催化剂的需求是当前从硝酸盐和亚硝酸盐来源合成氨的瓶颈。在本文中,我们介绍了以磷化铟量子点 (InP QD) 为光催化剂,从硝酸盐和亚硝酸盐离子选择性可见光驱动氨生产。催化铟位点的存在和通过催化剂-反应物相互作用的相互作用进行微环境调节,导致在可见光下高效和选择性地形成氨。在室温下可见光照射 2 小时内,以 ∼94% 的诱人收率生产出水相和气相氨。在阳光下也观察到了氨的良好形成,加强了 InP QD 光催化剂的转化前景。机理研究确定了竞争性析氢在直接硝酸盐还原中的作用可以忽略不计,证实了来自 InP QD 的光激发电载流子积极参与氨合成。动力学研究表明,在能量上具有挑战性的硝酸盐到亚硝酸盐的转化是速率确定步骤,随后的反应进行 ∼100% 转化以产生氨。一系列实验得出结论,水是 InP QD 光催化合成氨的质子源。我们的研究表明,基于 InP QD 的光催化剂的合理设计的核心和表面对开发超越 Haber-Bosch 工艺生产氨的可持续路线的影响。
更新日期:2024-11-18
中文翻译:

用可见光和量子点合成氨
从硝酸盐和亚硝酸盐来源光辅助合成氨是一种可持续的方法,可以减轻能源密集型 Haber-Bosch 工艺的负担。然而,选择性差和对紫外线活性光催化剂的需求是当前从硝酸盐和亚硝酸盐来源合成氨的瓶颈。在本文中,我们介绍了以磷化铟量子点 (InP QD) 为光催化剂,从硝酸盐和亚硝酸盐离子选择性可见光驱动氨生产。催化铟位点的存在和通过催化剂-反应物相互作用的相互作用进行微环境调节,导致在可见光下高效和选择性地形成氨。在室温下可见光照射 2 小时内,以 ∼94% 的诱人收率生产出水相和气相氨。在阳光下也观察到了氨的良好形成,加强了 InP QD 光催化剂的转化前景。机理研究确定了竞争性析氢在直接硝酸盐还原中的作用可以忽略不计,证实了来自 InP QD 的光激发电载流子积极参与氨合成。动力学研究表明,在能量上具有挑战性的硝酸盐到亚硝酸盐的转化是速率确定步骤,随后的反应进行 ∼100% 转化以产生氨。一系列实验得出结论,水是 InP QD 光催化合成氨的质子源。我们的研究表明,基于 InP QD 的光催化剂的合理设计的核心和表面对开发超越 Haber-Bosch 工艺生产氨的可持续路线的影响。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号