当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Magnetic Properties, and Hydrogen Evolution Reaction of a Butterfly-like Heterometallic Trinuclear [CuII2MnII] Cluster
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.inorgchem.4c03723 Chandan Sarkar, Aditi De, Subir Maji, Julia Kłak, Subrata Kundu, Manindranath Bera
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-18 , DOI: 10.1021/acs.inorgchem.4c03723 Chandan Sarkar, Aditi De, Subir Maji, Julia Kłak, Subrata Kundu, Manindranath Bera
|
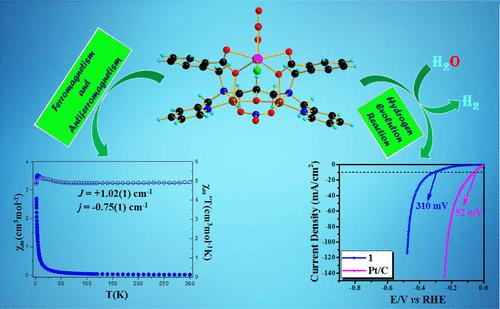
A novel heterometallic trinuclear cluster [CuII2MnII(cpdp)(NO3)2(Cl)] (1) has been designed and synthesized by employing a molecular library approach that uses CuCl2·2H2O and Mn(NO3)2·4H2O as inorganic metal salts and H3cpdp as a multifunctional organic scaffold (H3cpdp = N,N′-bis[2-carboxybenzomethyl]-N,N′-bis[2-pyridylmethyl]-1,3-diaminopropan-2-ol). This heterometallic cluster has emerged as an unusual ferromagnetic material and promising electrocatalyst for hydrogen evolution reaction (HER) in the domain of inorganic and materials chemistry. Crystal structure analysis establishes the structural arrangement of 1, revealing a butterfly-like topology with an unusual seven-coordinated Mn(II) center. Formation of this cluster is accomplished by a self-assembly process through functionalization of 1 with one μ2:η1:η1-nitrate and two μ2:η2:η1-benzoate groups via the CuII(μ2-NO3)CuII} and {CuII(μ2-O2CC6H5)MnII} linkages, respectively. Variable-temperature SQUID magnetometry revealed the coexistence of ferromagnetic and antiferromagnetic interactions in 1. The observed magnetic behavior in 1 is unexpected because of a large Cu–O–Mn angle with a value of 132.05°, indicating that the correlation between coupling constants and the structural parameters is a multifactor problem. This cluster shows excellent electrocatalytic performance for the HER attaining a current density of 10 mA/cm2 with a Tafel slope of 183 mV dec–1 at a 310 mV overpotential value. Essentially, cluster 1 shows exceptional electrochemical stability at ambient temperature, accompanied by minimal degradation of the current density as examined by chronoamperometric studies. Density functional theory calculations establish the mechanistic insight into the HER process, indicating that the CuII–OCO–MnII site is the active site for formation of molecular hydrogen.
中文翻译:

蝴蝶状异金属三核 [CuII2MnII] 团簇的设计、合成、磁性能和析氢反应
以CuCl2·2H2O和Mn(NO 3)2·4H2O为无机金属盐,以H 3 cpdp为多功能有机支架(H3cpdp = N,N′-双[2-羧基苯并甲基]-N,N′-双[2-吡啶甲基]-1,3-二氨基丙-2-醇)。这种异金属团簇已成为一种不常见的铁磁材料,是无机和材料化学领域析氢反应 (HER) 的有前途的电催化剂。晶体结构分析确定了 1 的结构排列,揭示了一个蝴蝶状拓扑结构,具有一个不寻常的七个配位 Mn(II) 中心。该簇的形成是通过自组装过程完成的,通过一个2:η1:η 1-硝酸盐基团和两个2:η2:η1-苯甲酸盐基团的功能化μ μ通过 CuII(μ2-NO 3)CuII} 和 {CuII(μ2-O 2CC6H5)MnII} 链接。变温 SQUID 磁力测量揭示了 1.在 1 中观察到的磁行为是出乎意料的,因为 Cu-O-Mn 角很大,值为 132.05°,表明耦合常数与结构参数之间的相关性是一个多因素问题。 该簇对 HER 表现出优异的电催化性能,在 310 mV 过电位值下达到 10 mA/cm2 的电流密度,塔菲尔斜率为 183 mV dec–1。从本质上讲,簇 1 在环境温度下表现出出色的电化学稳定性,并且通过计时安培研究检查的电流密度下降最小。密度泛函理论计算建立了对 HER 过程的机理见解,表明 Cu II-OCO-MnII 位点是形成分子氢的活性位点。
更新日期:2024-11-18
中文翻译:

蝴蝶状异金属三核 [CuII2MnII] 团簇的设计、合成、磁性能和析氢反应
以CuCl2·2H2O和Mn(NO 3)2·4H2O为无机金属盐,以H 3 cpdp为多功能有机支架(H3cpdp = N,N′-双[2-羧基苯并甲基]-N,N′-双[2-吡啶甲基]-1,3-二氨基丙-2-醇)。这种异金属团簇已成为一种不常见的铁磁材料,是无机和材料化学领域析氢反应 (HER) 的有前途的电催化剂。晶体结构分析确定了 1 的结构排列,揭示了一个蝴蝶状拓扑结构,具有一个不寻常的七个配位 Mn(II) 中心。该簇的形成是通过自组装过程完成的,通过一个2:η1:η 1-硝酸盐基团和两个2:η2:η1-苯甲酸盐基团的功能化μ μ通过 CuII(μ2-NO 3)CuII} 和 {CuII(μ2-O 2CC6H5)MnII} 链接。变温 SQUID 磁力测量揭示了 1.在 1 中观察到的磁行为是出乎意料的,因为 Cu-O-Mn 角很大,值为 132.05°,表明耦合常数与结构参数之间的相关性是一个多因素问题。 该簇对 HER 表现出优异的电催化性能,在 310 mV 过电位值下达到 10 mA/cm2 的电流密度,塔菲尔斜率为 183 mV dec–1。从本质上讲,簇 1 在环境温度下表现出出色的电化学稳定性,并且通过计时安培研究检查的电流密度下降最小。密度泛函理论计算建立了对 HER 过程的机理见解,表明 Cu II-OCO-MnII 位点是形成分子氢的活性位点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号