当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bacterial Biosynthesis of Nitrile-Containing Natural Products: Basis for Recognition of Diversified Substrates
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-17 , DOI: 10.1021/acscatal.4c06581 Ming Peng, Qiaoling Wu, Lele Ma, Zhao-Jie Teng, Xuben Hou, Hongjie Zhu, Jianhua Ju
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-17 , DOI: 10.1021/acscatal.4c06581 Ming Peng, Qiaoling Wu, Lele Ma, Zhao-Jie Teng, Xuben Hou, Hongjie Zhu, Jianhua Ju
|
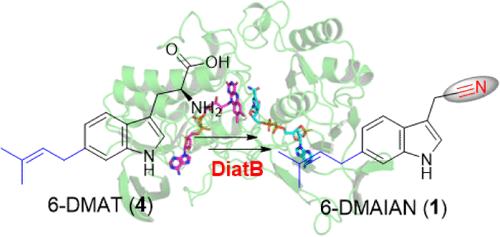
Nitrile-containing natural products, despite being a limited group of secondary metabolites, display remarkable structural and functional diversities. Aldoxime formation represents a crucial step in nitrile installation via the aldoxime-nitrile pathway although structural information regarding aldoxime formation is extremely limited. Here, we report the isolation of a nitrile compound 6-dimethylallylindole-3-acetonitrile (6-DMAIAN) and identify the aldoxime-forming enzyme gene diatB as a robust reporter for bacterial nitrile biosynthesis. We characterize the flavin-dependent monooxygenase DiatB and provide structural and mechanistic insights into the structural parameters dictating its substrate compatibilites. This enzyme initiates a nucleophilic attack on the amino group of the substrate 6-dimethylallyl-l-tryptophan (6-DMAT), resulting in formation of a transient aldoxime that precedes nitrile installation. Moreover, the DiatB recognition motif is elucidated shedding light on its substrate flexibility. We also apply bioinformatics analysis to examine the distribution and diversity of functional DiatB homologues across an array of potential nitrile-forming organisms. Given the activity of DiatB and its prevalence in secondary metabolite biosynthesis, our results provide important insight into what is, arguably, the most crucial and pivotal step in nitrile biosynthesis; these findings also suggest a promising enzymatic tool for nitrile drug design.
中文翻译:

含丁腈天然产物的细菌生物合成:识别多种底物的基础
含丁腈的天然产物尽管是一组有限的次生代谢产物,但表现出显着的结构和功能多样性。醛肟的形成代表了通过醛肟-腈途径进行丁腈安装的关键步骤,尽管有关醛肟形成的结构信息极其有限。在这里,我们报道了腈化合物 6-二甲基烯丙吲哚-3-乙腈 (6-DMAIAN) 的分离,并确定了醛肟形成酶基因 diatB 作为细菌腈生物合成的稳健报告基因。我们表征了黄素依赖性单加氧酶 DiatB,并提供了对决定其底物相容性的结构参数的结构和机制见解。该酶对底物 6-二甲基烯丙基-l-色氨酸 (6-DMAT) 的氨基发起亲核攻击,导致在腈安装之前形成瞬时醛肟。此外,阐明了 DiatB 识别基序,阐明了其底物的灵活性。我们还应用生物信息学分析来检查功能性 DiatB 同源物在一系列潜在的腈形成生物体中的分布和多样性。鉴于 DiatB 的活性及其在次生代谢物生物合成中的普遍性,我们的结果为可以说是丁腈生物合成中最关键和关键的步骤提供了重要的见解;这些发现还表明了一种很有前途的腈类药物设计的酶工具。
更新日期:2024-11-17
中文翻译:

含丁腈天然产物的细菌生物合成:识别多种底物的基础
含丁腈的天然产物尽管是一组有限的次生代谢产物,但表现出显着的结构和功能多样性。醛肟的形成代表了通过醛肟-腈途径进行丁腈安装的关键步骤,尽管有关醛肟形成的结构信息极其有限。在这里,我们报道了腈化合物 6-二甲基烯丙吲哚-3-乙腈 (6-DMAIAN) 的分离,并确定了醛肟形成酶基因 diatB 作为细菌腈生物合成的稳健报告基因。我们表征了黄素依赖性单加氧酶 DiatB,并提供了对决定其底物相容性的结构参数的结构和机制见解。该酶对底物 6-二甲基烯丙基-l-色氨酸 (6-DMAT) 的氨基发起亲核攻击,导致在腈安装之前形成瞬时醛肟。此外,阐明了 DiatB 识别基序,阐明了其底物的灵活性。我们还应用生物信息学分析来检查功能性 DiatB 同源物在一系列潜在的腈形成生物体中的分布和多样性。鉴于 DiatB 的活性及其在次生代谢物生物合成中的普遍性,我们的结果为可以说是丁腈生物合成中最关键和关键的步骤提供了重要的见解;这些发现还表明了一种很有前途的腈类药物设计的酶工具。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号