Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the Interplay of Protein Self‐Assembly and Covalent Bond Formation in Photo‐Crosslinked Silk Fibroin Hydrogels
Small ( IF 13.0 ) Pub Date : 2024-11-16 , DOI: 10.1002/smll.202407923 Hien A. Tran, Anton Maraldo, Trinh Thi‐Phuong Ho, Mai Thanh Thai, Quinn van Hilst, Habib Joukhdar, Marija Kordanovski, Jugal Kishore Sahoo, Onur Hartsuk, Miguel Santos, Steven G. Wise, David L. Kaplan, Thanh Nho Do, Kristopher A. Kilian, Khoon S. Lim, Jelena Rnjak‐Kovacina
Small ( IF 13.0 ) Pub Date : 2024-11-16 , DOI: 10.1002/smll.202407923 Hien A. Tran, Anton Maraldo, Trinh Thi‐Phuong Ho, Mai Thanh Thai, Quinn van Hilst, Habib Joukhdar, Marija Kordanovski, Jugal Kishore Sahoo, Onur Hartsuk, Miguel Santos, Steven G. Wise, David L. Kaplan, Thanh Nho Do, Kristopher A. Kilian, Khoon S. Lim, Jelena Rnjak‐Kovacina
|
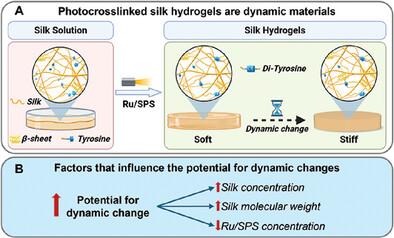
Covalent crosslinking of silk fibroin via native tyrosine residues has been extensively explored; however, while these materials are very promising for biomedical, optical, soft robotics, and sensor applications, their structure and mechanical properties are unstable over time. This instability results in spontaneous silk self‐assembly and stiffening over time, a process that is poorly understood. This study investigates the interplay between self‐assembly and di‐tyrosine bond formation in silk hydrogels photo‐crosslinked using ruthenium (Ru) and sodium persulfate (SPS) with visible light. The effects of silk concentration, molecular weight, Ru/SPS concentration, and solvent conditions are examined. The Ru/SPS system enables rapid crosslinking, achieving gelation within seconds and incorporating over 90% of silk into the network, even at very low protein concentrations (≥0.75% wt/v). A model emerges where silk self‐assembly both before and after crosslinking affects protein phase separation, mesoscale structure, and dynamic changes in the hydrogel network over time. Silk concentration has the greatest impact on hydrogel properties, with higher silk concentration hydrogels experiencing two orders of magnitude increase in stiffness within 1 week. This new understanding and ability to tune hydrogel properties and dynamic stiffening aids in developing advanced materials for 4D biofabrication, sensing, 3D cancer models, drug delivery, and soft robotics.
中文翻译:

探索光交联丝素蛋白水凝胶中蛋白质自组装和共价键形成的相互作用
丝素蛋白通过天然酪氨酸残基的共价交联已被广泛探索;然而,虽然这些材料在生物医学、光学、软机器人和传感器应用方面非常有前途,但它们的结构和机械性能会随着时间的推移而不稳定。这种不稳定性导致真丝自发地自组装并随着时间的推移而变硬,这一过程知之甚少。本研究在可见光下研究了使用钌 (Ru) 和过硫酸钠 (SPS) 光交联的丝水凝胶中自组装和二酪氨酸键形成之间的相互作用。检查了蚕丝浓度、分子量、Ru/SPS 浓度和溶剂条件的影响。Ru/SPS 系统可实现快速交联,在几秒钟内实现凝胶化,并将超过 90% 的丝掺入网络,即使在非常低的蛋白质浓度 (≥0.75% wt/v) 下也是如此。出现了一个模型,其中交联前后的丝自组装会影响蛋白质相分离、中尺度结构和水凝胶网络随时间的动态变化。蚕丝浓度对水凝胶性能的影响最大,蚕丝浓度较高的水凝胶在 1 周内的刚度增加了两个数量级。这种调整水凝胶特性和动态硬化的新理解和能力有助于开发用于 4D 生物制造、传感、3D 癌症模型、药物输送和软机器人的先进材料。
更新日期:2024-11-16
中文翻译:

探索光交联丝素蛋白水凝胶中蛋白质自组装和共价键形成的相互作用
丝素蛋白通过天然酪氨酸残基的共价交联已被广泛探索;然而,虽然这些材料在生物医学、光学、软机器人和传感器应用方面非常有前途,但它们的结构和机械性能会随着时间的推移而不稳定。这种不稳定性导致真丝自发地自组装并随着时间的推移而变硬,这一过程知之甚少。本研究在可见光下研究了使用钌 (Ru) 和过硫酸钠 (SPS) 光交联的丝水凝胶中自组装和二酪氨酸键形成之间的相互作用。检查了蚕丝浓度、分子量、Ru/SPS 浓度和溶剂条件的影响。Ru/SPS 系统可实现快速交联,在几秒钟内实现凝胶化,并将超过 90% 的丝掺入网络,即使在非常低的蛋白质浓度 (≥0.75% wt/v) 下也是如此。出现了一个模型,其中交联前后的丝自组装会影响蛋白质相分离、中尺度结构和水凝胶网络随时间的动态变化。蚕丝浓度对水凝胶性能的影响最大,蚕丝浓度较高的水凝胶在 1 周内的刚度增加了两个数量级。这种调整水凝胶特性和动态硬化的新理解和能力有助于开发用于 4D 生物制造、传感、3D 癌症模型、药物输送和软机器人的先进材料。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号