当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermochemical Correlations of Redox and Brønsted Sites on Bifunctional Polyoxometalate Clusters and Their Kinetic Consequences in Methanol-O2 Catalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-16 , DOI: 10.1021/acscatal.4c04745 Guangming Cai, Ya-Huei Cathy Chin
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-16 , DOI: 10.1021/acscatal.4c04745 Guangming Cai, Ya-Huei Cathy Chin
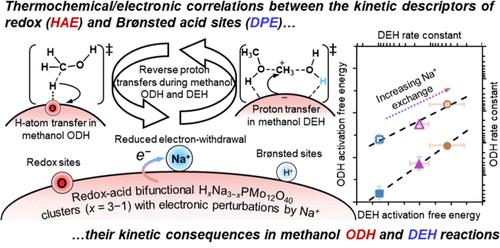
|
Kinetic interconnectivities of methanol oxidative dehydrogenation and dehydration are manifestation of the underlying thermochemical/electronic correlations between redox and Brønsted sites on bifunctional Keggin-type polyoxometalate (POM) phosphomolybdic acid clusters with their electronic properties perturbed by sodium cation exchange (HxNa3–xPMo, x = 3–0). As sodium exchange increases, activation free energies for the elementary C–H scission in methanol oxidative dehydrogenation, occurring at isolated redox sites (O*) or Brønsted acid-redox site pairs (OH/O*), and for the first-order C–O formation in methanol dehydration, occurring at Brønsted sites, increase proportionally within 10–11 kJ mol–1 at 433 K, while their activation enthalpies exhibit an inverse correlation. A Born–Haber thermochemical analysis reveals the reasons behind the site interconnectivities by establishing their respective kinetic-thermochemical relationships. The kinetically relevant C–H scission involves a late transition state, either [HOCH2···H···O*]‡ at O* or [OH···HOCH2···H···O*]‡ at OH/O*, with the transfer of an electron (e–) and a proton (H+) as an H atom (H•) from the methyl fragment to redox sites, where hydrogen addition energy (HAE), comprising the negative electron affinity (−EAPOM) and proton affinity (−PA) of POM clusters, is a kinetic descriptor. The parallel methanol C–O formation features a late carbocationic transition state, [(CH3OH···CH3+···H2O)···POM–]‡, involving proton transfer from POM clusters to adsorbed methanol species, where the deprotonation energy (DPE) of the Brønsted site serves as a kinetic descriptor. Notably, hydrogen addition energy decreases by ∼23 kJ mol–1, while deprotonation energy increases by 80–230 kJ mol–1, as sodium exchange increases. This slight negative thermochemical correlation arises from the inherent opposing proton transfers during redox (−PA) and Brønsted acid catalysis (DPE), modulated by the energetic effect of electron transfer (−EAPOM) upon sodium exchange on HxNa3–xPMo clusters (x = 3–1). The mechanistic interpretation and framework established here explicitly correlate the kinetic, thermochemical, and electronic properties of redox and Brønsted sites, offering insights into their intrinsic reactivity couplings, and are applicable to other bifunctional catalysts.
中文翻译:

双功能多金属氧酸盐簇上氧化还原位点和 Brønsted 位点的热化学相关性及其在甲醇-O 2 催化中的动力学后果
甲醇氧化脱氢和脱水的动力学互连性是双功能 Keggin 型多金属氧酸盐 (POM) 磷酸钼酸团簇上氧化还原位点和 Brønsted 位点之间潜在热化学/电子相关性的体现,其电子性质受到钠阳离子交换 (HxNa3–xPMo, x= 3–0)。随着钠交换的增加,甲醇氧化脱氢中发生在分离氧化还原位点 (O*) 或 Brønsted 酸-氧化还原位点对 (OH/O*) 的基本 C-H 断裂的活化自由能,以及发生在 Brønsted 位点的甲醇脱水中一级 C-O 形成的活化自由能,在 10–11 kJ mol–1 范围内成比例增加在 433 K 时,而它们的活化焓表现出负相关。Born-Haber 热化学分析通过建立它们各自的动力学-热化学关系来揭示站点互连性背后的原因。动力学相关的 C-H 断裂涉及晚期过渡态,要么 [HOCH2···H···O*]‡ 在 O* 或 [OH···霍赫2···H···OH/O* 的 O*]‡,电子 (e–) 和质子 (H+) 作为 H 原子 (H•) 从甲基片段转移到氧化还原位点,其中氢加成能 (HAE) 包括 POM 簇的负电子亲和力 (−EAPOM) 和质子亲和力 (−PA),是一个动力学描述符。 平行甲醇 C–O 形成具有晚期碳阳离子过渡态 [(CH3OH···CH3+···H2O)···POM–]‡,涉及质子从 POM 簇转移到吸附的甲醇物质,其中 Brønsted 位点的去质子化能 (DPE) 用作动力学描述符。值得注意的是,随着钠交换的增加,氢加成能降低 ∼23 kJ mol–1,而去质子化能增加 80–230 kJ mol–1。这种轻微的负热化学相关性源于氧化还原 (-PA) 和 Brønsted 酸催化 (DPE) 过程中固有的相反质子转移,受 HxNa 3-xPMo 簇 (x = 3-1) 上钠交换时电子转移 (-EAPOM) 的能量效应的调节。这里建立的机理解释和框架明确关联了氧化还原和 Brønsted 位点的动力学、热化学和电子性质,提供了对它们的本征反应性偶联的见解,并适用于其他双功能催化剂。
更新日期:2024-11-16
中文翻译:

双功能多金属氧酸盐簇上氧化还原位点和 Brønsted 位点的热化学相关性及其在甲醇-O 2 催化中的动力学后果
甲醇氧化脱氢和脱水的动力学互连性是双功能 Keggin 型多金属氧酸盐 (POM) 磷酸钼酸团簇上氧化还原位点和 Brønsted 位点之间潜在热化学/电子相关性的体现,其电子性质受到钠阳离子交换 (HxNa3–xPMo, x= 3–0)。随着钠交换的增加,甲醇氧化脱氢中发生在分离氧化还原位点 (O*) 或 Brønsted 酸-氧化还原位点对 (OH/O*) 的基本 C-H 断裂的活化自由能,以及发生在 Brønsted 位点的甲醇脱水中一级 C-O 形成的活化自由能,在 10–11 kJ mol–1 范围内成比例增加在 433 K 时,而它们的活化焓表现出负相关。Born-Haber 热化学分析通过建立它们各自的动力学-热化学关系来揭示站点互连性背后的原因。动力学相关的 C-H 断裂涉及晚期过渡态,要么 [HOCH2···H···O*]‡ 在 O* 或 [OH···霍赫2···H···OH/O* 的 O*]‡,电子 (e–) 和质子 (H+) 作为 H 原子 (H•) 从甲基片段转移到氧化还原位点,其中氢加成能 (HAE) 包括 POM 簇的负电子亲和力 (−EAPOM) 和质子亲和力 (−PA),是一个动力学描述符。 平行甲醇 C–O 形成具有晚期碳阳离子过渡态 [(CH3OH···CH3+···H2O)···POM–]‡,涉及质子从 POM 簇转移到吸附的甲醇物质,其中 Brønsted 位点的去质子化能 (DPE) 用作动力学描述符。值得注意的是,随着钠交换的增加,氢加成能降低 ∼23 kJ mol–1,而去质子化能增加 80–230 kJ mol–1。这种轻微的负热化学相关性源于氧化还原 (-PA) 和 Brønsted 酸催化 (DPE) 过程中固有的相反质子转移,受 HxNa 3-xPMo 簇 (x = 3-1) 上钠交换时电子转移 (-EAPOM) 的能量效应的调节。这里建立的机理解释和框架明确关联了氧化还原和 Brønsted 位点的动力学、热化学和电子性质,提供了对它们的本征反应性偶联的见解,并适用于其他双功能催化剂。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号