当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Insights into Hydrogen Peroxide Generation on Carbon Electrodes: Influence of Defects, Oxygen Functional Groups, and Alkali Metals in the Electrolyte
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c04734 André Olean-Oliveira, Najeeb Hasnain, Ricardo Martínez-Hincapié, Ulrich Hagemann, Adarsh Jain, Doris Segets, Ioannis Spanos, Viktor Čolić
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c04734 André Olean-Oliveira, Najeeb Hasnain, Ricardo Martínez-Hincapié, Ulrich Hagemann, Adarsh Jain, Doris Segets, Ioannis Spanos, Viktor Čolić
|
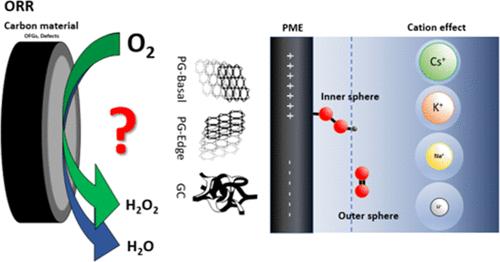
Hydrogen peroxide (H2O2) is an environmentally friendly oxidant, with production reaching 5.7 million tons by 2028 and a market size of USD 4.04 billion by 2029. Understanding the mechanism of oxygen reduction to H2O2 and the structure–activity relations on carbon materials is, therefore, of high significance for the more environmentally friendly synthesis of this important chemical. We have used oriented pyrolytic graphite (PG-edge and PG-basal) and glassy carbon (GC) as model electrodes to investigate the influence of carbon defects, oxygen-containing functional groups, and the presence of alkali metals on the activity and selectivity toward H2O2 production under acidic conditions. Electrochemical measurements, such as rotating ring disk electrode and electrochemical impedance spectroscopy, as well as in situ Raman spectroelectrochemistry indicated that PG-basal and GC electrodes preferentially form H2O2 as the product through the two-electron pathway via inner and outer sphere mechanisms, respectively. The mechanism is significantly affected by the potential of maximal entropy, which determines the position of species in the solution within the inner or outer Helmholtz plane. The influence of alkali cations (Li+, Na+, K+, and Cs+) on the oxygen reduction reaction of these model carbon electrodes was investigated. Large cations, e.g., K+ and Cs+, showed influence on the reaction intermediates and thus on the electrodes’ selectivity. The present study provides important insights and contributions to the fundamental aspects of hydrogen peroxide production in acidic conditions and further advancements in the development of metal-free carbon-based catalysts.
中文翻译:

碳电极上过氧化氢生成的电化学见解:电解质中缺陷、氧官能团和碱金属的影响
过氧化氢 (H2O2) 是一种环保型氧化剂,到 2028 年产量将达到 570 万吨,到 2029 年市场规模将达到 40.4 亿美元。因此,了解氧还原为 H2O2 的机理以及碳材料上的构效关系对于更环保地合成这种重要化学品具有重要意义。我们使用取向热解石墨 (PG-edge 和 PG-basal) 和玻碳 (GC) 作为模型电极,研究了碳缺陷、含氧官能团和碱金属的存在对酸性条件下对 H2O2 产生的活性和选择性的影响。电化学测量,如旋转环盘电极和电化学阻抗谱,以及原位拉曼光谱电化学表明,PG 基电极和 GC 电极分别通过内球和外球机制通过双电子途径优先形成 H2O2 作为产物。该机制受最大熵势的显着影响,最大熵的势决定了物质在内亥姆霍兹平面或外亥姆霍兹平面内溶液中的位置。研究了碱阳离子 (Li+、Na+、K+ 和 Cs+) 对这些模型碳电极氧还原反应的影响。大阳离子,例如 K+ 和 Cs +,对反应中间体有影响,从而对电极的选择性有影响。 本研究为酸性条件下过氧化氢生产的基本方面以及无金属碳基催化剂开发的进一步进展提供了重要的见解和贡献。
更新日期:2024-11-16
中文翻译:

碳电极上过氧化氢生成的电化学见解:电解质中缺陷、氧官能团和碱金属的影响
过氧化氢 (H2O2) 是一种环保型氧化剂,到 2028 年产量将达到 570 万吨,到 2029 年市场规模将达到 40.4 亿美元。因此,了解氧还原为 H2O2 的机理以及碳材料上的构效关系对于更环保地合成这种重要化学品具有重要意义。我们使用取向热解石墨 (PG-edge 和 PG-basal) 和玻碳 (GC) 作为模型电极,研究了碳缺陷、含氧官能团和碱金属的存在对酸性条件下对 H2O2 产生的活性和选择性的影响。电化学测量,如旋转环盘电极和电化学阻抗谱,以及原位拉曼光谱电化学表明,PG 基电极和 GC 电极分别通过内球和外球机制通过双电子途径优先形成 H2O2 作为产物。该机制受最大熵势的显着影响,最大熵的势决定了物质在内亥姆霍兹平面或外亥姆霍兹平面内溶液中的位置。研究了碱阳离子 (Li+、Na+、K+ 和 Cs+) 对这些模型碳电极氧还原反应的影响。大阳离子,例如 K+ 和 Cs +,对反应中间体有影响,从而对电极的选择性有影响。 本研究为酸性条件下过氧化氢生产的基本方面以及无金属碳基催化剂开发的进一步进展提供了重要的见解和贡献。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号