当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Electrochemical Mediator for Oxidative Multi-Site Proton Coupled Electron Transfer
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c05832 Tarisha Gupta, Yati, Sanyam, Anirban Mondal, Biswajit Mondal
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c05832 Tarisha Gupta, Yati, Sanyam, Anirban Mondal, Biswajit Mondal
|
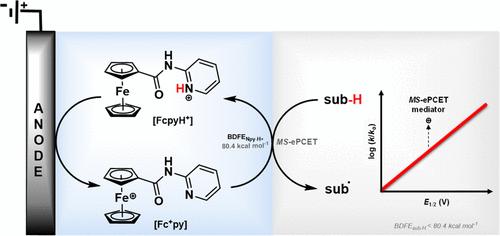
Proton-coupled electron transfer (PCET) allows a kinetically favorable pathway for electrochemical conversions. Inspired by this, an electrochemical mediator, N-pyridylferrocenecarboxamide (Fcpy), having site-separated electron and proton transfer sites and its analog are reported. The BDFE of the Fcpy mediator is estimated to be 80.4 kcal mol–1. As a proof-of-concept study, Hantzsch ester (HE) having a C–H BDFE of 70.70 kcal mol–1 has been electrochemically oxidized to yield 93% of the desired product. The computational data suggests an ET-PCET-PT process for the mediated HE oxidation with Fcpy. Further, the electrochemical HE oxidation kinetics is recorded for a series of ferrocene derivatives devoid of any Brønsted base and having different E1/2 and is compared with the Fcpy and its analog. The logarithm (rate) vs E1/2 for electrochemical HE oxidation shows a clear kinetic advantage for the multisite PCET mediators. Eyring analysis revealed crucial activation parameters for the MS-PCET mediator.
中文翻译:

用于氧化多位点质子耦合电子转移的分子电化学介质
质子耦合电子转移 (PCET) 为电化学转化提供了动力学上有利的途径。受此启发,报道了一种电化学介质 N-吡啶基二元二烯甲酰胺 (Fcpy),具有位点分离的电子和质子转移位点及其类似物。Fcpy 介质的 BDFE 估计为 80.4 kcal mol-1。作为一项概念验证研究,具有 70.70 kcal mol–1 的 C-H BDFE 的 Hantzsch 酯 (HE) 已被电化学氧化,可产生 93% 的所需产物。计算数据表明 Fcpy 介导的 HE 氧化的 ET-PCET-PT 过程。此外,记录了一系列不含任何 Brønsted 碱且具有不同 E1/2 的二茂铁衍生物的电化学 HE 氧化动力学,并与 Fcpy 及其类似物进行了比较。电化学 HE 氧化的对数(速率)与 E1/2 的关系表明,多位点 PCET 介质具有明显的动力学优势。Eyring 分析揭示了 MS-PCET 介质的关键激活参数。
更新日期:2024-11-16
中文翻译:

用于氧化多位点质子耦合电子转移的分子电化学介质
质子耦合电子转移 (PCET) 为电化学转化提供了动力学上有利的途径。受此启发,报道了一种电化学介质 N-吡啶基二元二烯甲酰胺 (Fcpy),具有位点分离的电子和质子转移位点及其类似物。Fcpy 介质的 BDFE 估计为 80.4 kcal mol-1。作为一项概念验证研究,具有 70.70 kcal mol–1 的 C-H BDFE 的 Hantzsch 酯 (HE) 已被电化学氧化,可产生 93% 的所需产物。计算数据表明 Fcpy 介导的 HE 氧化的 ET-PCET-PT 过程。此外,记录了一系列不含任何 Brønsted 碱且具有不同 E1/2 的二茂铁衍生物的电化学 HE 氧化动力学,并与 Fcpy 及其类似物进行了比较。电化学 HE 氧化的对数(速率)与 E1/2 的关系表明,多位点 PCET 介质具有明显的动力学优势。Eyring 分析揭示了 MS-PCET 介质的关键激活参数。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号